Babesiosis
- PMID: 25999229
- PMCID: PMC4458703
- DOI: 10.1016/j.idc.2015.02.008
Babesiosis
Abstract
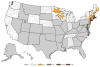
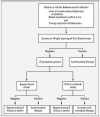
Babesiosis is caused by intraerythrocytic protozoan parasites that are transmitted by ticks, or less commonly through blood transfusion or transplacentally. Human babesiosis was first recognized in a splenectomized patient in Europe but most cases have been reported from the northeastern and upper midwestern United States in people with an intact spleen and no history of immune impairment. Cases are reported in Asia, Africa, Australia, Europe, and South America. Babesiosis shares many clinical features with malaria and can be fatal, particularly in the elderly and the immunocompromised.
Keywords: Apicomplexa; Babesia microti; Babesiosis; Erythrocyte; Protozoan; Tick; Transfusion.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- Skrabalo A, Deanovic A. Piroplasmosis in man: Report on a case. Doc Med Geogr Trop. 1957;9:11–16. - PubMed
-
- Vannier E, Krause PJ. Human babesiosis. New Engl J Med. 2012;366:2397–407. - PubMed
-
- Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38:1219–37. - PubMed
-
- Spielman A, Wilson ML, Levine JF, et al. Ecology of Ixodes dammini borne human babesiosis and Lyme disease. Ann Rev Entomol. 1985;30:439–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

