Nuclear Factor Erythroid 2-Related Factor 2 Drives Podocyte-Specific Expression of Peroxisome Proliferator-Activated Receptor γ Essential for Resistance to Crescentic GN
- PMID: 25999406
- PMCID: PMC4696572
- DOI: 10.1681/ASN.2014111080
Nuclear Factor Erythroid 2-Related Factor 2 Drives Podocyte-Specific Expression of Peroxisome Proliferator-Activated Receptor γ Essential for Resistance to Crescentic GN
Abstract
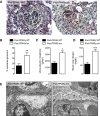
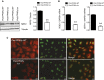
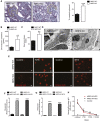
Necrotizing and crescentic rapidly progressive GN (RPGN) is a life-threatening syndrome characterized by a rapid loss of renal function. Evidence suggests that podocyte expression of the transcription factor peroxisome proliferator-activated receptor γ (PPARγ) may prevent podocyte injury, but the function of glomerular PPARγ in acute, severe inflammatory GN is unknown. Here, we observed marked loss of PPARγ abundance and transcriptional activity in glomerular podocytes in experimental RPGN. Blunted expression of PPARγ in podocyte nuclei was also found in kidneys from patients diagnosed with crescentic GN. Podocyte-specific Pparγ gene targeting accentuated glomerular damage, with increased urinary loss of albumin and severe kidney failure. Furthermore, a PPARγ gain-of-function approach achieved by systemic administration of thiazolidinedione (TZD) failed to prevent severe RPGN in mice with podocyte-specific Pparγ gene deficiency. In nuclear factor erythroid 2-related factor 2 (NRF2)-deficient mice, loss of podocyte PPARγ was observed at baseline. NRF2 deficiency markedly aggravated the course of RPGN, an effect that was partially prevented by TZD administration. Furthermore, delayed administration of TZD, initiated after the onset of RPGN, still alleviated the severity of experimental RPGN. These findings establish a requirement for the NRF2-PPARγ cascade in podocytes, and we suggest that these transcription factors have a role in augmenting the tolerance of glomeruli to severe immune-complex mediated injury. The NRF2-PPARγ pathway may be a therapeutic target for RPGN.
Keywords: focal segmental glomerulosclerosis; glomerular disease; glomerulonephritis; metabolism; podocyte; renal protection.
Copyright © 2016 by the American Society of Nephrology.
Figures











References
-
- Besse-Eschmann V, Le Hir M, Endlich N, Endlich K: Alteration of podocytes in a murine model of crescentic glomerulonephritis. Histochem Cell Biol 122: 139–149, 2004 - PubMed
-
- Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB: Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol 15: 61–67, 2004 - PubMed
-
- Le Hir M, Keller C, Eschmann V, Hähnel B, Hosser H, Kriz W: Podocyte bridges between the tuft and Bowman’s capsule: an early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol 12: 2060–2071, 2001 - PubMed
-
- Bariety J, Mandet C, Hill GS, Bruneval P: Parietal podocytes in normal human glomeruli. J Am Soc Nephrol 17: 2770–2780, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

