Proteotoxicity and cardiac dysfunction
- PMID: 25999425
- PMCID: PMC4443853
- DOI: 10.1161/CIRCRESAHA.116.305372
Proteotoxicity and cardiac dysfunction
Abstract
Baseline physiological function of the mammalian heart is under the constant threat of environmental or intrinsic pathological insults. Cardiomyocyte proteins are thus subject to unremitting pressure to function optimally, and this depends on them assuming and maintaining proper conformation. This review explores the multiple defenses a cell may use for its proteins to assume and maintain correct protein folding and conformation. There are multiple quality control mechanisms to ensure that nascent polypeptides are properly folded and mature proteins maintain their functional conformation. When proteins do misfold, either in the face of normal or pathological stimuli or because of intrinsic mutations or post-translational modifications, they must either be refolded correctly or recycled. In the absence of these corrective processes, they may become toxic to the cell. Herein, we explore some of the underlying mechanisms that lead to proteotoxicity. The continued presence and chronic accumulation of misfolded or unfolded proteins can be disastrous in cardiomyocytes because these misfolded proteins can lead to aggregation or the formation of soluble peptides that are proteotoxic. This in turn leads to compromised protein quality control and precipitating a downward spiral of the cell's ability to maintain protein homeostasis. Some underlying mechanisms are discussed and the therapeutic potential of interfering with proteotoxicity in the heart is explored.
Keywords: autophagy; proteasome; protein.
© 2015 American Heart Association, Inc.
Figures

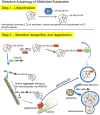
 ) binds ubiquitinated proteins for interaction with LC3 in a growing autophagosome, thus selectively targeting the protein for autophagic degradation after fusion with lysosomes. In addition, HDAC6 can bind ubiquitinated proteins through its ubiquitin binding domain (
) binds ubiquitinated proteins for interaction with LC3 in a growing autophagosome, thus selectively targeting the protein for autophagic degradation after fusion with lysosomes. In addition, HDAC6 can bind ubiquitinated proteins through its ubiquitin binding domain (
 ) and transport them in a retrograde fashion along microtubules by binding to the motor protein dynein through the dynein motor binding domain (
) and transport them in a retrograde fashion along microtubules by binding to the motor protein dynein through the dynein motor binding domain (
 ). These can be single misfolded species or soluble proto-aggregates. This event can happen many times, causing the proteins to coalesce and form aggresomes in perinuclear region of the cell. Aggresomal proteins can also be degraded by autophagy, and these cargo transport proteins may be involved in this process as well, particularly HDAC6 with known roles in mediating the autophagosome-lysosome fusion event.
). These can be single misfolded species or soluble proto-aggregates. This event can happen many times, causing the proteins to coalesce and form aggresomes in perinuclear region of the cell. Aggresomal proteins can also be degraded by autophagy, and these cargo transport proteins may be involved in this process as well, particularly HDAC6 with known roles in mediating the autophagosome-lysosome fusion event.


References
-
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

