CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia
- PMID: 25999455
- PMCID: PMC4481592
- DOI: 10.1182/blood-2014-12-580068
CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia
Abstract
Relapsed and refractory acute lymphoblastic leukemia (ALL) remains difficult to treat, with minimal improvement in outcomes seen in more than 2 decades despite advances in upfront therapy and improved survival for de novo ALL. Adoptive transfer of T cells engineered to express a chimeric antigen receptor (CAR) has emerged as a powerful targeted immunotherapy, showing striking responses in highly refractory populations. Complete remission (CR) rates as high as 90% have been reported in children and adults with relapsed and refractory ALL treated with CAR-modified T cells targeting the B-cell-specific antigen CD19. Distinct CAR designs across several studies have produced similar promising CR rates, an encouraging finding. Even more encouraging are durable remissions observed in some patients without additional therapy. Duration of remission and CAR-modified T-cell persistence require further study and more mature follow-up, but emerging data suggest these factors may distinguish CAR designs. Supraphysiologic T-cell proliferation, a hallmark of this therapy, contributes to both efficacy and the most notable toxicity, cytokine release syndrome (CRS), posing a unique challenge for toxicity management. This review will discuss the current landscape of CD19 CAR clinical trials, CRS pathophysiology and management, and remaining challenges.
© 2015 by The American Society of Hematology.
Figures
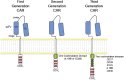

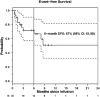
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

