Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology
- PMID: 25999512
- PMCID: PMC5658207
- DOI: 10.1126/science.aaa6566
Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology
Abstract
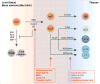
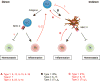
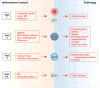
Innate lymphoid cells (ILCs) are a growing family of immune cells that mirror the phenotypes and functions of T cells. However, in contrast to T cells, ILCs do not express acquired antigen receptors or undergo clonal selection and expansion when stimulated. Instead, ILCs react promptly to signals from infected or injured tissues and produce an array of secreted proteins termed cytokines that direct the developing immune response into one that is adapted to the original insult. The complex cross-talk between microenvironment, ILCs, and adaptive immunity remains to be fully deciphered. Only by understanding these complex regulatory networks can the power of ILCs be controlled or unleashed in order to regulate or enhance immune responses in disease prevention and therapy.
Copyright © 2015, American Association for the Advancement of Science.
Figures

References
-
- Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3−LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493. - PubMed
-
- Adachi S, Yoshida H, Kataoka H, Nishikawa S. Three distinctive steps in Peyer’s patch formation of murine embryo. Int Immunol. 1997;9:507. - PubMed
-
- Satoh-Takayama N, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

