Application of dental nanomaterials: potential toxicity to the central nervous system
- PMID: 25999717
- PMCID: PMC4437601
- DOI: 10.2147/IJN.S79892
Application of dental nanomaterials: potential toxicity to the central nervous system
Abstract
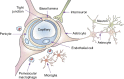
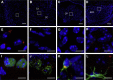
Nanomaterials are defined as materials with one or more external dimensions with a size of 1-100 nm. Such materials possess typical nanostructure-dependent properties (eg, chemical, biological, optical, mechanical, and magnetic), which may differ greatly from the properties of their bulk counterparts. In recent years, nanomaterials have been widely used in the production of dental materials, particularly in light polymerization composite resins and bonding systems, coating materials for dental implants, bioceramics, endodontic sealers, and mouthwashes. However, the dental applications of nanomaterials yield not only a significant improvement in clinical treatments but also growing concerns regarding their biosecurity. The brain is well protected by the blood-brain barrier (BBB), which separates the blood from the cerebral parenchyma. However, in recent years, many studies have found that nanoparticles (NPs), including nanocarriers, can transport through the BBB and locate in the central nervous system (CNS). Because the CNS may be a potential target organ of the nanomaterials, it is essential to determine the neurotoxic effects of NPs. In this review, possible dental nanomaterials and their pathways into the CNS are discussed, as well as related neurotoxicity effects underlying the in vitro and in vivo studies. Finally, we analyze the limitations of the current testing methods on the toxicological effects of nanomaterials. This review contributes to a better understanding of the nano-related risks to the CNS as well as the further development of safety assessment systems.
Keywords: central nervous system; dental; nanomaterials; risk assessment; testing methods; toxicity.
Figures

Comment in
-
Potential toxicity of dental nanomaterials to the central nervous system.Int J Nanomedicine. 2015 Sep 3;10:5593-4. doi: 10.2147/IJN.S91856. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26366079 Free PMC article. No abstract available.
-
Authors' reply.Int J Nanomedicine. 2015;10:5595-6. Int J Nanomedicine. 2015. PMID: 26594672 No abstract available.
References
-
- Bleeker EA, de Jong WH, Geertsma RE, et al. Considerations on the EU definition of a nanomaterial: science to support policy making. Regul Toxicol Pharm. 2013;65(1):119–125. - PubMed
-
- Juanola-Feliu E. The nanotechnology revolution in Barcelona: innovation and creativity by universities. Manage Int. 2009;13:111–123.
-
- Niu LN, Fang M, Jiao K, et al. Tetrapod-like zinc oxide whisker enhancement of resin composite. J Dent Res. 2010;89(7):746–750. - PubMed
-
- Memarzadeh K, Sharili AS, Huang J, Rawlinson SC, Allaker RP. Nanoparticulate zinc oxide as a coating material for orthopedic and dental implants. J Biomed Mater Res A. 2015;103(3):981–989. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

