Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer
- PMID: 25999946
- PMCID: PMC4422082
- DOI: 10.3389/fimmu.2015.00201
Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer
Abstract
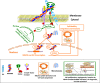
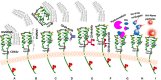
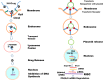
The glycosaminoglycan hyaluronan (HA), a major component of extracellular matrices, and cell surface receptors of HA have been proposed to have pivotal roles in cell proliferation, migration, and invasion, which are necessary for inflammation and cancer progression. CD44 and receptor for HA-mediated motility (RHAMM) are the two main HA-receptors whose biological functions in human and murine inflammations and tumor cells have been investigated comprehensively. HA was initially considered to be only an inert component of connective tissues, but is now known as a "dynamic" molecule with a constant turnover in many tissues through rapid metabolism that involves HA molecules of various sizes: high molecular weight HA (HMW HA), low molecular weight HA, and oligosaccharides. The intracellular signaling pathways initiated by HA interactions with CD44 and RHAMM that lead to inflammatory and tumorigenic responses are complex. Interestingly, these molecules have dual functions in inflammations and tumorigenesis. For example, the presence of CD44 is involved in initiation of arthritis, while the absence of CD44 by genetic deletion in an arthritis mouse model increases rather than decreases disease severity. Similar dual functions of CD44 exist in initiation and progression of cancer. RHAMM overexpression is most commonly linked to cancer progression, whereas loss of RHAMM is associated with malignant peripheral nerve sheath tumor growth. HA may similarly perform dual functions. An abundance of HMW HA can promote malignant cell proliferation and development of cancer, whereas antagonists to HA-CD44 signaling inhibit tumor cell growth in vitro and in vivo by interfering with HMW HA-CD44 interaction. This review describes the roles of HA interactions with CD44 and RHAMM in inflammatory responses and tumor development/progression, and how therapeutic strategies that block these key inflammatory/tumorigenic processes may be developed in rodent and human diseases.
Keywords: CD44; RHAMM; cancer; hyaluronan; inflammation.
Figures

References
-
- Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med (2002) 8:850–5. - PubMed
-
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest (2000) 106:349–60.10.1172/JCI10272 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

