Shigella manipulates host immune responses by delivering effector proteins with specific roles
- PMID: 25999954
- PMCID: PMC4423471
- DOI: 10.3389/fimmu.2015.00219
Shigella manipulates host immune responses by delivering effector proteins with specific roles
Abstract
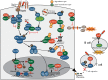
The intestinal epithelium deploys multiple defense systems against microbial infection to sense bacterial components and danger alarms, as well as to induce intracellular signal transduction cascades that trigger both the innate and the adaptive immune systems, which are pivotal for bacterial elimination. However, many enteric bacterial pathogens, including Shigella, deliver a subset of virulence proteins (effectors) via the type III secretion system (T3SS) that enable bacterial evasion from host immune systems; consequently, these pathogens are able to efficiently colonize the intestinal epithelium. In this review, we present and select recently discovered examples of interactions between Shigella and host immune responses, with particular emphasis on strategies that bacteria use to manipulate inflammatory outputs of host-cell responses such as cell death, membrane trafficking, and innate and adaptive immune responses.
Keywords: Shigella; effector; inflammation; innate immunity.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

