Myositis-specific autoantibodies: detection and clinical associations
- PMID: 26000115
- PMCID: PMC4389074
- DOI: 10.1007/s13317-011-0018-8
Myositis-specific autoantibodies: detection and clinical associations
Abstract
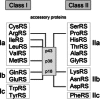
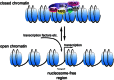
In recent years, the detection and characterization of (novel) autoantibodies is becoming increasingly important for the early diagnosis of autoimmune diseases. The idiopathic inflammatory myopathies (IIM, also indicated with myositis) are a group of systemic autoimmune disorders that involve inflammation and weakness of skeletal muscles. One of the hallmarks is the infiltration of inflammatory cells in muscle tissues. A number of myositis-specific autoantibodies have been identified and these may be associated with distinct IIM subclasses and clinical symptoms. Here, we review all myositis-specific autoantibodies identified today as well as their target proteins, together with their clinical associations in IIM patients. Post-translational modifications that might be associated with the generation of autoantibodies and the development of the disease are discussed as well. In addition, we describe well established autoantibody detection techniques that are currently being used in diagnostic laboratories, as well as novel multiplexed methods. The latter techniques provide great opportunities for the simultaneous detection of distinct autoantibodies, but may also contribute to the identification of novel autoantibody profiles, which may have additional diagnostic and prognostic value. The ongoing characterization of novel autoantibody specificities emphasizes the complexity of processes involved in the development of such autoimmune diseases.
Keywords: Autoantibody detection; Autoantigen; IIM; Multiplex assays; Myositis-specific autoantibodies; Post-translational modification.
Figures
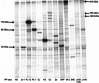
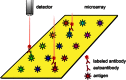
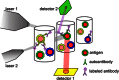

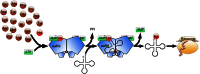

References
-
- Mammen AL. Dermatomyositis and polymyositis: clinical presentation, autoantibodies, and pathogenesis. Ann N Y Acad Sci. 2010;1184:134–153. - PubMed
-
- Walker UA. Imaging tools for the clinical assessment of idiopathic inflammatory myositis. Curr Opin Rheumatol. 2008;20:656–661. - PubMed
-
- Tozzoli R. Recent advances in diagnostic technologies and their impact in autoimmune diseases. Autoimmun Rev. 2007;6:334–340. - PubMed
-
- Sharp V, Utz PJ. Technology insight: can autoantibody profiling improve clinical practice? Nat Clin Pract Rheumatol. 2007;3:96–103. - PubMed
-
- Betteridge Z, Gunawardena H, North J, et al. Anti-synthetase syndrome: a new autoantibody to phenylalanyl transfer RNA synthetase (anti-Zo) associated with polymyositis and interstitial pneumonia. Rheumatology (Oxford) 2007;46:1005–1008. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

