Ciclamilast ameliorates adjuvant-induced arthritis in a rat model
- PMID: 26000303
- PMCID: PMC4426775
- DOI: 10.1155/2015/786104
Ciclamilast ameliorates adjuvant-induced arthritis in a rat model
Abstract
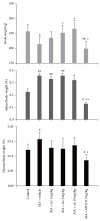
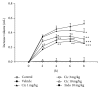
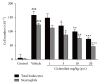
We assessed the effect of a novel and selective phosphodiesterase 4 (PDE4) inhibitor, ciclamilast, on chronic inflammation in adjuvant-induced arthritis (AIA), a rat model of rheumatoid arthritis (RA), and acute inflammation in the rat and mouse model of carrageenan-induced paw edema and peritonitis. Our results showed that daily oral administration of ciclamilast at 1, 3, and 10 mg/kg dose-dependently inhibited the increase in hind paw volume of rats with AIA. The inhibition of paw edema was associated with inhibition of both the production of cytokines such as TNF-α, IL-1β, and IL-6 and cell infiltration assessed in subcutaneous paw tissue. Moreover, there was significantly less tissue destruction in the ciclamilast-treated rats compared to the vehicle-treated rats, as assessed by radiographic analysis and histopathological evaluation. In the two acute inflammation models, ciclamilast inhibited carrageenan-induced paw edema in rats and inflammatory cell migration into the peritoneal cavity in mice in a dose-dependent manner. These results not only suggest that ciclamilast, as a disease-modifying antirheumatic drug (DMARD), can attenuate RA but also provide proof of principle that a PDE4 inhibitor may be useful for the treatment of arthritis.
Figures



References
-
- Singh J. A., Furst D. E., Bharat A., et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care & Research. 2012;64(5):625–639. doi: 10.1002/acr.21641. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

