Biomarkers and cognitive endpoints to optimize trials in Alzheimer's disease
- PMID: 26000325
- PMCID: PMC4435707
- DOI: 10.1002/acn3.192
Biomarkers and cognitive endpoints to optimize trials in Alzheimer's disease
Abstract
Objective: To find the combination of candidate biomarkers and cognitive endpoints to maximize statistical power and minimize cost of clinical trials of healthy elders at risk for cognitive decline due to Alzheimer's disease.
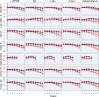
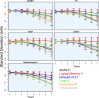
Methods: Four-hundred and twelve cognitively normal participants were followed over 7 years. Nonlinear methods were used to estimate the longitudinal trajectories of several cognitive outcomes including delayed memory recall, executive function, processing speed, and several cognitive composites by subgroups selected on the basis of biomarkers, including APOE-ε4 allele carriers, cerebrospinal fluid biomarkers (Aβ 42, total tau, and phosphorylated tau), and those with small hippocampi.
Results: Derived cognitive composites combining Alzheimer's Disease Assessment Scale (ADAS)-cog scores with additional delayed memory recall and executive function components captured decline more robustly across biomarker groups than any measure of a single cognitive domain or ADAS-cog alone. Substantial increases in power resulted when including only participants positive for three or more biomarkers in simulations of clinical trials.
Interpretation: Clinical trial power may be improved by selecting participants on the basis of amyloid and neurodegeneration biomarkers and carefully tailoring primary cognitive endpoints to reflect the expected decline specific to these individuals.
Figures

References
-
- Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–321. - PubMed
-
- Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Aβ42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. - PubMed
-
- Hyman BT, Marzloff K, Arriagada PV. The lack of accumulation of senile plaques or amyloid burden in Alzheimer's disease suggests a dynamic balance between amyloid deposition and resolution. J Neuropathol Exp Neurol. 1993;52:594–600. - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

