The Roles of ROS and Caspases in TRAIL-Induced Apoptosis and Necroptosis in Human Pancreatic Cancer Cells
- PMID: 26000607
- PMCID: PMC4441514
- DOI: 10.1371/journal.pone.0127386
The Roles of ROS and Caspases in TRAIL-Induced Apoptosis and Necroptosis in Human Pancreatic Cancer Cells
Abstract
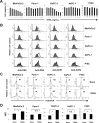
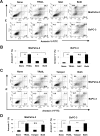
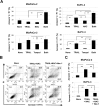
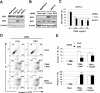
Death signaling provided by tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) can induce death in cancer cells with little cytotoxicity to normal cells; this cell death has been thought to involve caspase-dependent apoptosis. Reactive oxygen species (ROS) are also mediators that induce cell death, but their roles in TRAIL-induced apoptosis have not been elucidated fully. In the current study, we investigated ROS and caspases in human pancreatic cancer cells undergoing two different types of TRAIL-induced cell death, apoptosis and necroptosis. TRAIL treatment increased ROS in two TRAIL-sensitive pancreatic cancer cell lines, MiaPaCa-2 and BxPC-3, but ROS were involved in TRAIL-induced apoptosis only in MiaPaCa-2 cells. Unexpectedly, inhibition of ROS by either N-acetyl-L-cysteine (NAC), a peroxide inhibitor, or Tempol, a superoxide inhibitor, increased the annexin V-/propidium iodide (PI)+ early necrotic population in TRAIL-treated cells. Additionally, both necrostatin-1, an inhibitor of receptor-interacting protein kinase 1 (RIP1), and siRNA-mediated knockdown of RIP3 decreased the annexin V-/PI+ early necrotic population after TRAIL treatment. Furthermore, an increase in early apoptosis was induced in TRAIL-treated cancer cells under inhibition of either caspase-2 or -9. Caspase-2 worked upstream of caspase-9, and no crosstalk was observed between ROS and caspase-2/-9 in TRAIL-treated cells. Together, these results indicate that ROS contribute to TRAIL-induced apoptosis in MiaPaCa-2 cells, and that ROS play an inhibitory role in TRAIL-induced necroptosis of MiaPaCa-2 and BxPC-3 cells, with caspase-2 and -9 playing regulatory roles in this process.
Conflict of interest statement
Figures


References
-
- Ashkenazi A, Dixit VM (1999) Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 11: 255–60. - PubMed
-
- Almasan A, Ashkenazi A (2003) Cytokine & Growth Factor Reviews 14: 337–48. - PubMed
-
- Almasan A, Ashkenazi A (2003) Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev 14:337–348. - PubMed
-
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997) An antagonist decpy receptor and a death domain-containing receptor for TRAIL. Science 277:815–818. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

