Evaluating molecular cobalt complexes for the conversion of N2 to NH3
- PMID: 26001022
- PMCID: PMC4603980
- DOI: 10.1021/acs.inorgchem.5b00645
Evaluating molecular cobalt complexes for the conversion of N2 to NH3
Abstract
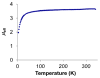
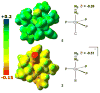
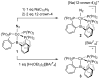
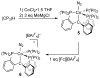
Well-defined molecular catalysts for the reduction of N2 to NH3 with protons and electrons remain very rare despite decades of interest and are currently limited to systems featuring molybdenum or iron. This report details the synthesis of a molecular cobalt complex that generates superstoichiometric yields of NH3 (>200% NH3 per Co-N2 precursor) via the direct reduction of N2 with protons and electrons. While the NH3 yields reported herein are modest by comparison to those of previously described iron and molybdenum systems, they intimate that other metals are likely to be viable as molecular N2 reduction catalysts. Additionally, a comparison of the featured tris(phosphine)borane Co-N2 complex with structurally related Co-N2 and Fe-N2 species shows how remarkably sensitive the N2 reduction performance of potential precatalysts is. These studies enable consideration of the structural and electronic effects that are likely relevant to N2 conversion activity, including the π basicity, charge state, and geometric flexibility.
Figures

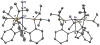

References
-
- Smil V. Enriching the Earth. MIT Press; Cambridge: 2001.
-
- Hidai M, Takahashi T, Yokotake I, Uchida Y. Chem Lett. 1980:645.
-
- Yamamoto A, Miura Y, Ito T, Chen H, Iri K, Ozawa F, Miki K, Sei T, Tanaka N, Kasai N. Organometallics. 1983;2:1429.
-
- Fryzuk MD. Acc Chem Res. 2009;42:127. - PubMed
- Schrock RR. Angew Chem Int Ed. 2008;47:5512. - PubMed
- Chirik PJ. Dalton Trans. 2007:16. - PubMed
- Peters JC, Mehn MP. In: Activation of Small Molecules: Organometallic and Bioinorganic Perspectives. Tolman WB, editor. Wiley-VCH; New York: 2006. pp. 81–119.
- Crossland JL, Tyler DR. Coord Chem Rev. 2010;254:1883.
- Chatt J, Dilworth JR, Richards RL. Chem Rev. 1978;78:589.
- Siedschlag RB, Bernales V, Vogiatzis KD, Planas N, Clouston LJ, Bill E, Gagliardi L, Lu CC. J Am Chem Soc. 2015;137:4638. - PubMed
-
- Laplaza CE, Cummins CC. Science. 1995;268:861. - PubMed
- Zanotti-Gerosa A, Solari E, Giannini L, Floriani C, Chiesi-Villa A, Rizzoli C. J Am Chem Soc. 1998;120:437.
- Nikiforov GB, Vidyaratne I, Gambarotta S, Korobkov I. Angew Chem Int Ed. 2009;48:7415. - PubMed
- Vidyaratne I, Crewdson P, Lefebvre E, Gambarotta S. Inorg Chem. 2007;46:8836. - PubMed
- Clentsmith GKB, Bates VME, Hitchcock PB, Cloke FGN. J Am Chem Soc. 1999;121:10444.
- Hebden TJ, Schrock RR, Takase MK, Müller P. Chem Commun. 2012;48:1851. - PubMed
- Rodriguez MM, Bill E, Brennessel WW, Holland PL. Science. 2011;334:780. - PMC - PubMed
- Curley JJ, Cook TR, Reece SY, Müller P, Cummins CC. J Am Chem Soc. 2008;130:9394. - PubMed
- Fryzuk MD, Kozak CM, Bowdridge MR, Patrick BO, Rettig SJ. J Am Chem Soc. 2002;124:8389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

