Micro/Nano gas sensors: a new strategy towards in-situ wafer-level fabrication of high-performance gas sensing chips
- PMID: 26001035
- PMCID: PMC5377049
- DOI: 10.1038/srep10507
Micro/Nano gas sensors: a new strategy towards in-situ wafer-level fabrication of high-performance gas sensing chips
Abstract
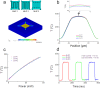
Nano-structured gas sensing materials, in particular nanoparticles, nanotubes, and nanowires, enable high sensitivity at a ppb level for gas sensors. For practical applications, it is highly desirable to be able to manufacture such gas sensors in batch and at low cost. We present here a strategy of in-situ wafer-level fabrication of the high-performance micro/nano gas sensing chips by naturally integrating microhotplatform (MHP) with nanopore array (NPA). By introducing colloidal crystal template, a wafer-level ordered homogenous SnO2 NPA is synthesized in-situ on a 4-inch MHP wafer, able to produce thousands of gas sensing units in one batch. The integration of micromachining process and nanofabrication process endues micro/nano gas sensing chips at low cost, high throughput, and with high sensitivity (down to ~20 ppb), fast response time (down to ~1 s), and low power consumption (down to ~30 mW). The proposed strategy of integrating MHP with NPA represents a versatile approach for in-situ wafer-level fabrication of high-performance micro/nano gas sensors for real industrial applications.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Hagleitner C. et al. Smart single-chip gas sensor microsystem. Nature 414, 293–296 (2001). - PubMed
-
- Wang Z. L. Functional oxide nanobelts: Materials, properties and potential applications in nanosystems and biotechnology. Annu. Rev. Phys. Chem. 55, 159–196 (2004). - PubMed
-
- Kolmakov A. & Moskovits M. Chemical sensing and catalysis by one-dimensional metal-oxide nanostructures. Annu. Rev. Mater. Res. 34, 151–180 (2004).
-
- Wang Y. D., Djerdj I., Antonietti M. & Smarsly B. Polymer-Assisted Generation of Antimony-Doped SnO2 Nanoparticles with High Crystallinity for Application in Gas Sensors. Small 4, 1656–1660 (2008). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

