Finger Prick Dried Blood Spots for HIV Viral Load Measurement in Field Conditions in Zimbabwe
- PMID: 26001044
- PMCID: PMC4441418
- DOI: 10.1371/journal.pone.0126878
Finger Prick Dried Blood Spots for HIV Viral Load Measurement in Field Conditions in Zimbabwe
Abstract
Background: In the context of a community-randomized trial of antiretrovirals for HIV prevention and treatment among sex workers in Zimbabwe (the SAPPH-IRe trial), we will measure the proportion of women with HIV viral load (VL) above 1000 copies/mL ("VL>1000") as our primary endpoint. We sought to characterize VL assay performance by comparing results from finger prick dried blood spots (DBS) collected in the field with plasma samples, to determine whether finger prick DBS is an acceptable sample for VL quantification in the setting.
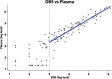
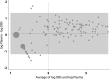
Methods: We collected whole blood from a finger prick onto filter paper and plasma samples using venipuncture from women in two communities. VL quantification was run on samples in parallel using NucliSENS EasyQ HIV-1 v2.0. Our trial outcome is the proportion of women with VL>1000, consistent with WHO guidelines relating to regimen switching. We therefore focused on this cut-off level for assessing sensitivity and specificity. Results were log transformed and the mean difference and standard deviation calculated, and correlation between VL quantification across sample types was evaluated.
Results: A total of 149 HIV-positive women provided DBS and plasma samples; 56 (63%) reported being on antiretroviral therapy. VL ranged from undetectable-6.08 log10 using DBS and undetectable-6.40 log10 using plasma. The mean difference in VL (plasma-DBS) was 0.077 log10 (95%CI = 0.025-0.18 log10; standard deviation = 0.63 log10,). 78 (52%) DBS and 87 (58%) plasma samples had a VL>1000. Based on plasma 'gold-standard', DBS sensitivity for detection of VL>1000 was 87.4%, and specificity was 96.8%.
Conclusion: There was generally good agreement between DBS and plasma VL for detection of VL>1000. Overall, finger prick DBS appeared to be an acceptable sample for classifying VL as above or below 1000 copies/mL using the NucliSENS assay.
Conflict of interest statement
Figures
References
-
- Cowan FM, Davey C, Napierala Mavedzenge S, Mushati P, Mtetwa S, Chiyaka T, et al. editors Estimation of the HIV care cascade for female sex workers in Zimbabwe: baseline results of the SAPPH-Ire trial. 20th International AIDS Conference; 2014; Melbourne, Australia.
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach Geneva: WHO; 2013. - PubMed
-
- World Health Organisation. Technical and operational considerations for implementing HIV viral load testing: interim technical update. Geneva: July 2014.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

