High resolution structural characterization of Aβ42 amyloid fibrils by magic angle spinning NMR
- PMID: 26001057
- PMCID: PMC4623963
- DOI: 10.1021/jacs.5b03997
High resolution structural characterization of Aβ42 amyloid fibrils by magic angle spinning NMR
Abstract
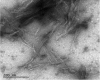
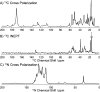
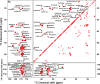
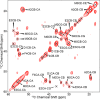
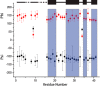
The presence of amyloid plaques composed of amyloid beta (Aβ) fibrils is a hallmark of Alzheimer's disease (AD). The Aβ peptide is present as several length variants with two common alloforms consisting of 40 and 42 amino acids, denoted Aβ1-40 and Aβ1-42, respectively. While there have been numerous reports that structurally characterize fibrils of Aβ1-40, very little is known about the structure of amyloid fibrils of Aβ1-42, which are considered the more toxic alloform involved in AD. We have prepared isotopically (13)C/(15)N labeled AβM01-42 fibrils in vitro from recombinant protein and examined their (13)C-(13)C and (13)C-(15)N magic angle spinning (MAS) NMR spectra. In contrast to several other studies of Aβ fibrils, we observe spectra with excellent resolution and a single set of chemical shifts, suggesting the presence of a single fibril morphology. We report the initial structural characterization of AβM01-42 fibrils utilizing (13)C and (15)N shift assignments of 38 of the 43 residues, including the backbone and side chains, obtained through a series of cross-polarization based 2D and 3D (13)C-(13)C, (13)C-(15)N MAS NMR experiments for rigid residues along with J-based 2D TOBSY experiments for dynamic residues. We find that the first ∼5 residues are dynamic and most efficiently detected in a J-based TOBSY spectrum. In contrast, residues 16-42 are easily observed in cross-polarization experiments and most likely form the amyloid core. Calculation of ψ and φ dihedral angles from the chemical shift assignments indicate that 4 β-strands are present in the fibril's secondary structure.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

