Fyn-phosphorylated PIKE-A binds and inhibits AMPK signaling, blocking its tumor suppressive activity
- PMID: 26001218
- PMCID: PMC4815978
- DOI: 10.1038/cdd.2015.66
Fyn-phosphorylated PIKE-A binds and inhibits AMPK signaling, blocking its tumor suppressive activity
Abstract
The AMP-activated protein kinase, a key regulator of energy homeostasis, has a critical role in metabolic disorders and cancers. AMPK is mainly regulated by cellular AMP and phosphorylation by upstream kinases. Here, we show that PIKE-A binds to AMPK and blocks its tumor suppressive actions, which are mediated by tyrosine kinase Fyn. PIKE-A directly interacts with AMPK catalytic alpha subunit and impairs T172 phosphorylation, leading to repression of its kinase activity on the downstream targets. Mutation of Fyn phosphorylation sites on PIKE-A, depletion of Fyn, or pharmacological inhibition of Fyn blunts the association between PIKE-A and AMPK, resulting in loss of its inhibitory effect on AMPK. Cell proliferation and oncogenic assays demonstrate that PIKE-A antagonizes tumor suppressive actions of AMPK. In human glioblastoma samples, PIKE-A expression inversely correlates with the p-AMPK levels, supporting that PIKE-A negatively regulates AMPK activity in cancers. Thus, our findings provide additional layer of molecular regulation of the AMPK signaling pathway in cancer progression.
Figures





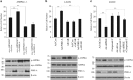

References
-
- Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem 2007; 282: 32539–32548. - PubMed
-
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 1996; 271: 27879–27887. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

