Prevalence of Antibodies Against JC Virus in Patients With Refractory Crohn's Disease and Effects of Natalizumab Therapy
- PMID: 26001336
- PMCID: PMC4795937
- DOI: 10.1016/j.cgh.2015.05.022
Prevalence of Antibodies Against JC Virus in Patients With Refractory Crohn's Disease and Effects of Natalizumab Therapy
Abstract
Background & aims: Natalizumab, a humanized antibody against the α4 integrin subunit, effectively induces and maintains remission in patients with Crohn's disease (CD) refractory to conventional treatments. Progressive multifocal leukoencephalopathy is a rare but fatal brain infection caused by John Cunningham (JC) virus and has been associated with natalizumab use. We assessed the prevalence of and risk factors for antibodies to JC virus in serum of patients with refractory CD who were candidates for, or already were receiving, natalizumab. We also assessed the effects of natalizumab treatment of these patients.
Methods: In a retrospective study, we analyzed clinical charts from 191 patients with CD (74 males; mean age, 38.7 y; mean duration of disease, 14.9 y) tested for serum JC virus antibody from December 2012 through May 2014 at 2 medical centers in the United States. We calculated JC virus antibody prevalence and compared the characteristics of patients who tested negative vs those who tested positive, to identify risk factors. We also assessed the rate of subsequent natalizumab use, surgery, and seroconversion during natalizumab therapy.
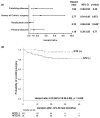
Results: A total of 129 of the patients (67.5%) tested positive for serum JC virus antibody. Multivariate analysis showed that past use of thiopurine was a risk factor for testing positive for JC virus antibody (odds ratio, 7.8; 95% confidence interval, 2.0-30.4; P = .003). Twenty-two of the patients who tested negative for JC virus antibody (35.5%) and 16 of the 129 patients who tested positive (12.4%) had been treated with natalizumab. Cox regression analysis determined that natalizumab use was the only factor associated with avoiding subsequent surgery (hazard ratio, 0.23; 95% confidence interval, 0.06-0.98). Seroconversion (from testing negative to positive for JC virus antibody) occurred in 1 of the 22 patients (4.5%) who initially tested negative during natalizumab therapy.
Conclusions: The prevalence of CD patients exposed to JC virus is comparable with that of the general population. In this retrospective study, prior thiopurine use was associated with an increased risk for testing positive for JC virus antibody. Natalizumab use reduced the risk of subsequent surgery.
Keywords: Biologic; IBD; Inflammatory Bowel Disease; PML; Thiopurine.
Copyright © 2015 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Natalizumab for Crohn's Disease: Down but Not Out.Clin Gastroenterol Hepatol. 2015 Nov;13(11):1926-8. doi: 10.1016/j.cgh.2015.07.020. Epub 2015 Jul 18. Clin Gastroenterol Hepatol. 2015. PMID: 26196443 No abstract available.
-
Failure to Account for Immortal Person Time Likely Accounts for the Apparent Reduction in Surgery Among Natalizumab Users.Clin Gastroenterol Hepatol. 2016 May;14(5):776. doi: 10.1016/j.cgh.2015.11.008. Epub 2015 Nov 22. Clin Gastroenterol Hepatol. 2016. PMID: 26614877 No abstract available.
-
Reply.Clin Gastroenterol Hepatol. 2016 May;14(5):776-7. doi: 10.1016/j.cgh.2016.02.002. Epub 2016 Feb 4. Clin Gastroenterol Hepatol. 2016. PMID: 26853161 No abstract available.
References
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. - PubMed
-
- Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006;41:10–6. - PubMed
-
- Sakuraba A, Sato T, Kamada N, et al. Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology. 2009;137:1736–45. - PubMed
-
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12(Suppl 1):S3–9. - PubMed
-
- Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. 2004;126:402–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

