Design, antiviral and cytostatic properties of isoxazolidine-containing amonafide analogues
- PMID: 26001344
- PMCID: PMC7126999
- DOI: 10.1016/j.bmc.2015.04.079
Design, antiviral and cytostatic properties of isoxazolidine-containing amonafide analogues
Abstract
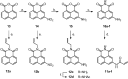
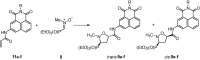
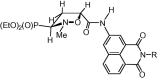
A novel series of 5-arylcarbamoyl- and 5-arylmethyl-2-methylisoxazolidin-3-yl-3-phosphonates have been synthesized via cycloaddition of N-methyl-C-(diethoxyphosphoryl)nitrone with N-substituted naphthalimide acrylamides and N-allylnaphthalimides. All cis- and trans-isoxazolidine phosphonates obtained herein were assessed for antiviral activity against a broad range of DNA and RNA viruses. Isoxazolidines trans-9d and trans-9f exhibited the highest activity (EC50=8.9μM) toward cytomegalovirus. Compounds cis- and trans-9d as well as cis- and trans-9f were found potent against HSV and Vaccinia viruses (EC50 in the 45-58μM range), whereas isoxazolidines 10a and 10d suppressed replication of Coxsackie B4 and Punta Toro viruses (EC50 in the 45-73μM range). Antiproliferative evaluation of all obtained isoxazolidines revealed the promising activity of cis-9b, cis-9d, trans-9d, cis-9e, trans-9e, cis-9f and trans-9f toward tested cancer cell lines with IC50 in the 1.1-19μM range.
Keywords: Antiviral; Cytostatic; Isoxazolidines; Naphthalimides; Phosphonates.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
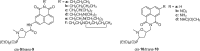
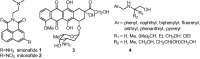
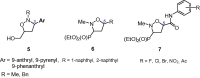
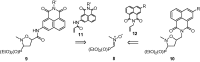

Similar articles
-
Novel 5-arylcarbamoyl-2-methylisoxazolidin-3-yl-3-phosphonates as nucleotide analogues.Nucleosides Nucleotides Nucleic Acids. 2014;33(8):552-82. doi: 10.1080/15257770.2014.909046. Nucleosides Nucleotides Nucleic Acids. 2014. PMID: 25009989
-
Isoxazolidine Conjugates of N3-Substituted 6-Bromoquinazolinones-Synthesis, Anti-Varizella-Zoster Virus, and Anti-Cytomegalovirus Activity.Molecules. 2018 Jul 28;23(8):1889. doi: 10.3390/molecules23081889. Molecules. 2018. PMID: 30060562 Free PMC article.
-
New Isoxazolidine-Conjugates of Quinazolinones-Synthesis, Antiviral and Cytostatic Activity.Molecules. 2016 Jul 22;21(7):959. doi: 10.3390/molecules21070959. Molecules. 2016. PMID: 27455228 Free PMC article.
-
Recent advances in the synthesis of cyclic 5'-nornucleoside phosphonate analogues.Carbohydr Res. 2018 Jun 30;463:47-106. doi: 10.1016/j.carres.2018.04.009. Epub 2018 Apr 28. Carbohydr Res. 2018. PMID: 29772449 Review.
-
Phosphonomethoxyalkyl analogs of nucleotides.Curr Pharm Des. 2003;9(31):2567-92. doi: 10.2174/1381612033453668. Curr Pharm Des. 2003. PMID: 14529543 Review.
Cited by
-
Stereoselective Synthesis of Isoxazolidines via Copper-Catalyzed Alkene Diamination.ACS Catal. 2017 Jul 7;7(7):4775-4779. doi: 10.1021/acscatal.7b01362. Epub 2017 Jun 15. ACS Catal. 2017. PMID: 29755827 Free PMC article.
-
1,8-naphthalimide-based DNA intercalators and anticancer agents: a systematic review.Mol Divers. 2025 Jun 18. doi: 10.1007/s11030-025-11251-1. Online ahead of print. Mol Divers. 2025. PMID: 40533630 Review.
-
An Overview of Naphthylimide as Specific Scaffold for New Drug Discovery.Molecules. 2024 Sep 24;29(19):4529. doi: 10.3390/molecules29194529. Molecules. 2024. PMID: 39407459 Free PMC article. Review.
-
Design and synthesis of DNA-intercalative naphthalimide-benzothiazole/cinnamide derivatives: cytotoxicity evaluation and topoisomerase-IIα inhibition.Medchemcomm. 2018 Nov 8;10(1):72-79. doi: 10.1039/c8md00395e. eCollection 2019 Jan 1. Medchemcomm. 2018. PMID: 30774856 Free PMC article.
-
Design, Synthesis and Bioactivities of Novel Isoxazole-Containing Pyrazole Oxime Derivatives.Molecules. 2017 Nov 27;22(12):2000. doi: 10.3390/molecules22122000. Molecules. 2017. PMID: 29186906 Free PMC article.
References
-
- Hendry L.B., Mahesh V.B., Bransome E.D., Jr., Ewing D.E. Mutat. Res. 2007;623:53. - PubMed
-
- Martínez R., Chacón-García L. Curr. Med. Chem. 2005;12:127. - PubMed
-
- Braña M.F., Cacho M., Gradillas A., de Pascual-Teresa B., Ramos A. Curr. Pharm. Design. 2001;7:1745. - PubMed
-
- Lerman L.S. J. Mol. Biol. 1961;3:18. - PubMed
-
- Hogan M., Dattagupta N., Crothers D.M. Biochemistry. 1979;18:280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

