Omega-3 fatty acids, lipid rafts, and T cell signaling
- PMID: 26001374
- PMCID: PMC4654711
- DOI: 10.1016/j.ejphar.2015.03.091
Omega-3 fatty acids, lipid rafts, and T cell signaling
Abstract
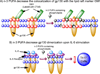
n-3 polyunsaturated fatty acids (PUFA) have been shown in many clinical studies to attenuate inflammatory responses. Although inflammatory responses are orchestrated by a wide spectrum of cells, CD4(+) T cells play an important role in the etiology of many chronic inflammatory diseases such as inflammatory bowel disease and obesity. In light of recent concerns over the safety profiles of non-steroidal anti-inflammatory drugs (NSAIDs), alternatives such as bioactive nutraceuticals are becoming more attractive. In order for these agents to be accepted into mainstream medicine, however, the mechanisms by which nutraceuticals such as n-3 PUFA exert their anti-inflammatory effects must be fully elucidated. Lipid rafts are nanoscale, dynamic domains in the plasma membrane that are formed through favorable lipid-lipid (cholesterol, sphingolipids, and saturated fatty acids) and lipid-protein (membrane-actin cytoskeleton) interactions. These domains optimize the clustering of signaling proteins at the membrane to facilitate efficient cell signaling which is required for CD4(+) T cell activation and differentiation. This review summarizes novel emerging data documenting the ability of n-3 PUFA to perturb membrane-cytoskeletal structure and function in CD4(+) T cells. An understanding of these underlying mechanisms will provide a rationale for the use of n-3 PUFA in the treatment of chronic inflammation.
Keywords: Lipid rafts; Omega-3 fatty acids; T cell activation; T cell differentiation.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Polyunsaturated fatty acids in the modulation of T-cell signalling.Prostaglandins Leukot Essent Fatty Acids. 2010 Apr-Jun;82(4-6):179-87. doi: 10.1016/j.plefa.2010.02.023. Epub 2010 Mar 1. Prostaglandins Leukot Essent Fatty Acids. 2010. PMID: 20189788 Review.
-
Long-chain n-3 fatty acids in lipid rafts: implications for anti-inflammatory effects.J Cardiovasc Med (Hagerstown). 2007 Sep;8 Suppl 1:S30-3. doi: 10.2459/01.JCM.0000289277.10675.e8. J Cardiovasc Med (Hagerstown). 2007. PMID: 17876195 Review.
-
n-3 polyunsaturated fatty acids suppress CD4(+) T cell proliferation by altering phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] organization.Biochim Biophys Acta. 2016 Jan;1858(1):85-96. doi: 10.1016/j.bbamem.2015.10.009. Epub 2015 Oct 23. Biochim Biophys Acta. 2016. PMID: 26476105 Free PMC article.
-
Polyunsaturated fatty acids interfere with formation of the immunological synapse.J Leukoc Biol. 2005 May;77(5):680-8. doi: 10.1189/jlb.1104687. Epub 2005 Feb 9. J Leukoc Biol. 2005. PMID: 15703198
-
Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance.Biochim Biophys Acta. 2015 Apr;1851(4):469-84. doi: 10.1016/j.bbalip.2014.08.010. Epub 2014 Aug 20. Biochim Biophys Acta. 2015. PMID: 25149823 Review.
Cited by
-
Upper Respiratory Tract Infections in Sport and the Immune System Response. A Review.Biology (Basel). 2021 Apr 23;10(5):362. doi: 10.3390/biology10050362. Biology (Basel). 2021. PMID: 33922542 Free PMC article. Review.
-
Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction.Nutrients. 2022 Mar 8;14(6):1137. doi: 10.3390/nu14061137. Nutrients. 2022. PMID: 35334794 Free PMC article. Review.
-
Linoleic acid supplementation of cell culture media influences the phospholipid and lipid profiles of human reconstructed adipose tissue.PLoS One. 2019 Oct 22;14(10):e0224228. doi: 10.1371/journal.pone.0224228. eCollection 2019. PLoS One. 2019. PMID: 31639818 Free PMC article.
-
The Role of Cytosolic Lipid Droplets in Hepatitis C Virus Replication, Assembly, and Release.Biomed Res Int. 2023 Apr 14;2023:5156601. doi: 10.1155/2023/5156601. eCollection 2023. Biomed Res Int. 2023. PMID: 37090186 Free PMC article. Review.
-
n-3 PUFA poor seafood consumption is associated with higher risk of gout, whereas n-3 PUFA rich seafood is not: NHANES 2007-2016.Front Nutr. 2023 Apr 4;10:1075877. doi: 10.3389/fnut.2023.1075877. eCollection 2023. Front Nutr. 2023. PMID: 37081920 Free PMC article.
References
-
- Arrington JL, McMurray DN, Switzer KC, Fan YY, Chapkin RS. Docosahexaenoic acid suppresses function of the CD28 costimulatory membrane receptor in primary murine and Jurkat T cells. J.Nutr. 2001;131:1147–1153. - PubMed
-
- Balamuth F, Leitenberg D, Unternaehrer J, Mellman I, Bottomly K. Distinct patterns of membrane microdomain partitioning in Th1 and th2 cells. Immunity. 2001;15:729–738. - PubMed
-
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
-
- Betz UA, Muller W. Regulated expression of gp130 and IL-6 receptor alpha chain in T cell maturation and activation. Int. Immunol. 1998;10:1175–1184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

