Improving response inhibition systems in frontotemporal dementia with citalopram
- PMID: 26001387
- PMCID: PMC5412666
- DOI: 10.1093/brain/awv133
Improving response inhibition systems in frontotemporal dementia with citalopram
Abstract
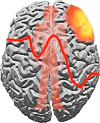
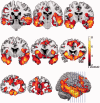
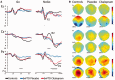
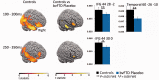
Disinhibition is a cardinal feature of the behavioural variant of frontotemporal dementia, presenting as impulsive and impetuous behaviours that are often difficult to manage. The options for symptomatic treatments are limited, but a potential target for therapy is the restoration of serotonergic function, which is both deficient in behavioural variant frontotemporal dementia and closely associated with inhibitory control. Based on preclinical studies and psychopharmacological interventions in other disorders, we predicted that inhibition would be associated with the right inferior frontal gyrus and dependent on serotonin. Using magnetoencephalography and electroencephalography of a Go-NoGo paradigm, we investigated the neural basis of behavioural disinhibition in behavioural variant frontotemporal dementia and the effect of selective serotonin reuptake inhibition on the neural systems for response inhibition. In a randomized double-blinded placebo-controlled crossover design study, 12 patients received either a single 30 mg dose of citalopram or placebo. Twenty age-matched healthy controls underwent the same magnetoencephalography/electroencephalography protocol on one session without citalopram, providing normative data for this task. In the control group, successful NoGo trials evoked two established indices of successful response inhibition: the NoGo-N2 and NoGo-P3. Both of these components were significantly attenuated by behavioural variant frontotemporal dementia. Cortical sources associated with successful inhibition in control subjects were identified in the right inferior frontal gyrus and anterior temporal lobe, which have been strongly associated with behavioural inhibition in imaging and lesion studies. These sources were impaired by behavioural variant frontotemporal dementia. Critically, citalopram enhanced the NoGo-P3 signal in patients, relative to placebo treatment, and increased the evoked response in the right inferior frontal gyrus. Voxel-based morphometry confirmed significant atrophy of inferior frontal gyrus, alongside insular, orbitofrontal and temporal cortex in our patient cohort. Together, these data suggest that the dysfunctional prefrontal cortical systems underlying response inhibition deficits in behavioural variant frontotemporal dementia can be partially restored by increasing serotonergic neurotransmission. The results support a translational neuroscience approach to impulsive neurological disorders and indicate the potential for symptomatic treatment of behavioural variant frontotemporal dementia including serotonergic strategies to improve disinhibition.media-1vid110.1093/brain/awv133_video_abstractawv133_video_abstract.
Keywords: citalopram; frontotemporal dementia; magnetoencephalography; response inhibition; voxel-based morphometry.
© The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
Comment in
- Neurodegener Dis Manag. 2015 Oct;5(5):383-4
Similar articles
-
Selective serotonin reuptake inhibition modulates response inhibition in Parkinson's disease.Brain. 2014 Apr;137(Pt 4):1145-55. doi: 10.1093/brain/awu032. Epub 2014 Feb 27. Brain. 2014. PMID: 24578545 Free PMC article. Clinical Trial.
-
Reorganization of cortical oscillatory dynamics underlying disinhibition in frontotemporal dementia.Brain. 2018 Aug 1;141(8):2486-2499. doi: 10.1093/brain/awy176. Brain. 2018. PMID: 29992242 Free PMC article.
-
Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia.Am J Geriatr Psychiatry. 2012 Sep;20(9):789-97. doi: 10.1097/JGP.0b013e31823033f3. Am J Geriatr Psychiatry. 2012. PMID: 21878805 Clinical Trial.
-
Fair play: social norm compliance failures in behavioural variant frontotemporal dementia.Brain. 2016 Jan;139(Pt 1):204-16. doi: 10.1093/brain/awv315. Epub 2015 Oct 26. Brain. 2016. PMID: 26503957
-
A neuroanatomical and cognitive model of impaired social behaviour in frontotemporal dementia.Brain. 2024 Jun 3;147(6):1953-1966. doi: 10.1093/brain/awae040. Brain. 2024. PMID: 38334506 Free PMC article. Review.
Cited by
-
Antidepressant use and cognitive decline in patients with dementia: a national cohort study.BMC Med. 2025 Feb 25;23(1):82. doi: 10.1186/s12916-025-03851-3. BMC Med. 2025. PMID: 39994788 Free PMC article.
-
Noradrenaline for progressive supranuclear palsy syndromes (NORAPS): a randomised, double-blind, placebo-controlled, crossover Phase IIb clinical trial evaluating the efficacy and safety of oral atomoxetine for treating cognitive and behavioural changes in people with progressive supranuclear palsy syndromes in the UK.BMJ Open. 2025 Jul 28;15(7):e099577. doi: 10.1136/bmjopen-2025-099577. BMJ Open. 2025. PMID: 40730397 Free PMC article.
-
Neuroinflammation as a Common Feature of Neurodegenerative Disorders.Front Pharmacol. 2019 Sep 12;10:1008. doi: 10.3389/fphar.2019.01008. eCollection 2019. Front Pharmacol. 2019. PMID: 31572186 Free PMC article. Review.
-
Managing cognition in progressive supranuclear palsy.Neurodegener Dis Manag. 2016 Dec;6(6):499-508. doi: 10.2217/nmt-2016-0027. Epub 2016 Nov 23. Neurodegener Dis Manag. 2016. PMID: 27879155 Free PMC article. Review.
-
Predicting loss of independence and mortality in frontotemporal lobar degeneration syndromes.J Neurol Neurosurg Psychiatry. 2021 Jul;92(7):737-744. doi: 10.1136/jnnp-2020-324903. Epub 2021 Feb 9. J Neurol Neurosurg Psychiatry. 2021. PMID: 33563798 Free PMC article.
References
-
- Albert J, López-Martín S, Hinojosa JA, Carretié L. Spatiotemporal characterization of response inhibition. Neuroimage 2013; 76: 272–81. - PubMed
-
- Almeida S, Glahn DC, Argyropoulos SV, Frangou S. Acute citalopram administration may disrupt contextual information processing in healthy males. Eur Psychiatry 2010; 25: 87–91. - PubMed
-
- Anderson IM, Clark L, Elliott R, Kulkarni B, Williams SR, Deakin JF. 5-HT(2C) receptor activation by m-chlorophenylpiperazine detected in humans with fMRI. Neuroreport 2002; 13: 1547–51. - PubMed
-
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 2003; 6: 115–6. - PubMed
-
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci 2004; 8: 170–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

