GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration
- PMID: 26001423
- PMCID: PMC4497834
- DOI: 10.1016/j.cmet.2015.05.010
GDF11 Increases with Age and Inhibits Skeletal Muscle Regeneration
Abstract
Age-related frailty may be due to decreased skeletal muscle regeneration. The role of TGF-β molecules myostatin and GDF11 in regeneration is unclear. Recent studies showed an age-related decrease in GDF11 and that GDF11 treatment improves muscle regeneration, which were contrary to prior studies. We now show that these recent claims are not reproducible and the reagents previously used to detect GDF11 are not GDF11 specific. We develop a GDF11-specific immunoassay and show a trend toward increased GDF11 levels in sera of aged rats and humans. GDF11 mRNA increases in rat muscle with age. Mechanistically, GDF11 and myostatin both induce SMAD2/3 phosphorylation, inhibit myoblast differentiation, and regulate identical downstream signaling. GDF11 significantly inhibited muscle regeneration and decreased satellite cell expansion in mice. Given early data in humans showing a trend for an age-related increase, GDF11 could be a target for pharmacologic blockade to treat age-related sarcopenia.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
H.N.N. and A.S.B. declare no conflicts.
Figures
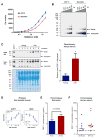
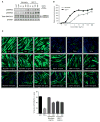
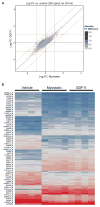
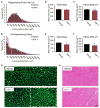

Comment in
-
GDF11 and the Mythical Fountain of Youth.Cell Metab. 2015 Jul 7;22(1):54-6. doi: 10.1016/j.cmet.2015.05.009. Epub 2015 May 20. Cell Metab. 2015. PMID: 26003784
Similar articles
-
Reduced Circulating GDF11 Is Unlikely Responsible for Age-Dependent Changes in Mouse Heart, Muscle, and Brain.Endocrinology. 2015 Nov;156(11):3885-8. doi: 10.1210/en.2015-1628. Epub 2015 Sep 15. Endocrinology. 2015. PMID: 26372181
-
The role of GDF11 in aging and skeletal muscle, cardiac and bone homeostasis.Crit Rev Biochem Mol Biol. 2019 Apr;54(2):174-183. doi: 10.1080/10409238.2019.1610722. Crit Rev Biochem Mol Biol. 2019. PMID: 31144559 Review.
-
Is Growth Differentiation Factor 11 a Realistic Therapeutic for Aging-Dependent Muscle Defects?Circ Res. 2016 Apr 1;118(7):1143-50; discussion 1150. doi: 10.1161/CIRCRESAHA.116.307962. Circ Res. 2016. PMID: 27034276 Free PMC article.
-
GDF11 promotes osteogenesis as opposed to MSTN, and follistatin, a MSTN/GDF11 inhibitor, increases muscle mass but weakens bone.Proc Natl Acad Sci U S A. 2020 Mar 3;117(9):4910-4920. doi: 10.1073/pnas.1916034117. Epub 2020 Feb 18. Proc Natl Acad Sci U S A. 2020. PMID: 32071240 Free PMC article.
-
The Growth Differentiation Factor 11 (GDF11) and Myostatin (MSTN) in tissue specific aging.Mech Ageing Dev. 2017 Jun;164:108-112. doi: 10.1016/j.mad.2017.04.009. Epub 2017 May 1. Mech Ageing Dev. 2017. PMID: 28472635 Review.
Cited by
-
The central role of muscle stem cells in regenerative failure with aging.Nat Med. 2015 Aug;21(8):854-62. doi: 10.1038/nm.3918. Nat Med. 2015. PMID: 26248268 Free PMC article. Review.
-
Turning back time with emerging rejuvenation strategies.Nat Cell Biol. 2019 Jan;21(1):32-43. doi: 10.1038/s41556-018-0206-0. Epub 2019 Jan 2. Nat Cell Biol. 2019. PMID: 30602763 Free PMC article. Review.
-
Functional replacement of myostatin with GDF-11 in the germline of mice.Skelet Muscle. 2022 Mar 15;12(1):7. doi: 10.1186/s13395-022-00290-z. Skelet Muscle. 2022. PMID: 35287700 Free PMC article.
-
GDF11 induces differentiation and apoptosis and inhibits migration of C17.2 neural stem cells via modulating MAPK signaling pathway.PeerJ. 2018 Sep 4;6:e5524. doi: 10.7717/peerj.5524. eCollection 2018. PeerJ. 2018. PMID: 30202652 Free PMC article.
-
Development of Myostatin Inhibitory d-Peptides to Enhance the Potency, Increasing Skeletal Muscle Mass in Mice.ACS Med Chem Lett. 2022 Feb 14;13(3):492-498. doi: 10.1021/acsmedchemlett.1c00705. eCollection 2022 Mar 10. ACS Med Chem Lett. 2022. PMID: 35300091 Free PMC article.
References
-
- Bass J, Oldham J, Sharma M, Kambadur R. Growth factors controlling muscle development. Domest Anim Endocrinol. 1999;17:191–197. - PubMed
-
- Beggs ML, Nagarajan R, Taylor-Jones JM, Nolen G, Macnicol M, Peterson CA. Alterations in the TGFbeta signaling pathway in myogenic progenitors with age. Aging Cell. 2004;3:353–361. - PubMed
-
- Gamer LW, Cox KA, Small C, Rosen V. Gdf11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb. Dev Biol. 2001;229:407–420. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

