DICOM for Clinical Research: PACS-Integrated Electronic Data Capture in Multi-Center Trials
- PMID: 26001521
- PMCID: PMC4570903
- DOI: 10.1007/s10278-015-9802-8
DICOM for Clinical Research: PACS-Integrated Electronic Data Capture in Multi-Center Trials
Abstract
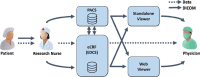
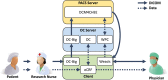
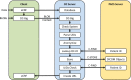
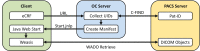
Providing surrogate endpoints in clinical trials, medical imaging has become increasingly important in human-centered research. Nowadays, electronic data capture systems (EDCS) are used but binary image data is integrated insufficiently. There exists no structured way, neither to manage digital imaging and communications in medicine (DICOM) data in EDCS nor to interconnect EDCS with picture archiving and communication systems (PACS). Manual detours in the trial workflow yield errors, delays, and costs. In this paper, requirements for a DICOM-based system interconnection of EDCS and research PACS are analysed. Several workflow architectures are compared. Optimized for multi-center trials, we propose an entirely web-based solution integrating EDCS, PACS, and DICOM viewer, which has been implemented using the open source projects OpenClinica, DCM4CHEE, and Weasis, respectively. The EDCS forms the primary access point. EDCS to PACS interchange is integrated seamlessly on the data and the context levels. DICOM data is viewed directly from the electronic case report form (eCRF), while PACS-based management is hidden from the user. Data privacy is ensured by automatic de-identification and re-labelling with study identifiers. Our concept is evaluated on a variety of 13 DICOM modalities and transfer syntaxes. We have implemented the system in an ongoing investigator-initiated trial (IIT), where five centers have recruited 24 patients so far, performing decentralized computed tomography (CT) screening. Using our system, the chief radiologist is reading DICOM data directly from the eCRF. Errors and workflow processing time are reduced. Furthermore, an imaging database is built that may support future research.
Keywords: Clinical trial; Digital Imaging and Communications in Medicine (DICOM); Open source; PACS; Systems integration; Workflow.
Figures

Similar articles
-
Digital Imaging and Electronic Data Capture in Multi-Center Clinical Trials.Stud Health Technol Inform. 2015;216:930. Stud Health Technol Inform. 2015. PMID: 26262232
-
CAD-PACS integration tool kit based on DICOM secondary capture, structured report and IHE workflow profiles.Comput Med Imaging Graph. 2007 Jun-Jul;31(4-5):346-52. doi: 10.1016/j.compmedimag.2007.02.015. Epub 2007 Mar 26. Comput Med Imaging Graph. 2007. PMID: 17386997
-
The case for RIS/PACS integration.Radiol Manage. 2002 May-Jun;24(3):24-9. Radiol Manage. 2002. PMID: 12080929
-
[Patient-centered image and data management in radiation oncology].Strahlenther Onkol. 2009 Jan;185(1):1-7. doi: 10.1007/s00066-009-1857-3. Epub 2009 Feb 18. Strahlenther Onkol. 2009. PMID: 19224141 Review. German.
-
Integration of computer-aided diagnosis/detection (CAD) results in a PACS environment using CAD-PACS toolkit and DICOM SR.Int J Comput Assist Radiol Surg. 2009 Jun;4(4):317-29. doi: 10.1007/s11548-009-0297-y. Epub 2009 Apr 15. Int J Comput Assist Radiol Surg. 2009. PMID: 20033579 Review.
Cited by
-
A Cloud-Ready Architecture for Shared Medical Imaging Repository.J Digit Imaging. 2020 Dec;33(6):1487-1498. doi: 10.1007/s10278-020-00373-7. J Digit Imaging. 2020. PMID: 32666366 Free PMC article.
-
Interoperable slide microscopy viewer and annotation tool for imaging data science and computational pathology.Nat Commun. 2023 Mar 22;14(1):1572. doi: 10.1038/s41467-023-37224-2. Nat Commun. 2023. PMID: 36949078 Free PMC article.
-
Smartphone imaging repository: a novel method for creating a CT image bank.Trials. 2023 Jan 20;24(1):46. doi: 10.1186/s13063-022-07052-8. Trials. 2023. PMID: 36670459 Free PMC article.
-
Head and Neck Cancer Adaptive Radiation Therapy (ART): Conceptual Considerations for the Informed Clinician.Semin Radiat Oncol. 2019 Jul;29(3):258-273. doi: 10.1016/j.semradonc.2019.02.008. Semin Radiat Oncol. 2019. PMID: 31027643 Free PMC article. Review.
-
Analysis of DICOM Image Compression Alternative Using Huffman Coding.J Healthc Eng. 2019 Jun 17;2019:5810540. doi: 10.1155/2019/5810540. eCollection 2019. J Healthc Eng. 2019. PMID: 31316743 Free PMC article.
References
-
- Miller CG, Krasnow J, Schwartz LH. Medical Imaging in Clinical Trials. London: Springer London; 2014.
-
- Fenstermacher D, Street C, McSherry T, Nayak V, Overby C, Feldman M: The cancer biomedical informatics Grid (caBIGTM). In: IEEE Engineering in Medicine and Biology 27th Annual Conference; 2005, p 743–746 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

