A porcine reproductive and respiratory syndrome virus (PRRSV) vaccine candidate based on the fusion protein of PRRSV glycoprotein 5 and the Toll-like Receptor-5 agonist Salmonella Typhimurium FljB
- PMID: 26001608
- PMCID: PMC4489122
- DOI: 10.1186/s12917-015-0439-0
A porcine reproductive and respiratory syndrome virus (PRRSV) vaccine candidate based on the fusion protein of PRRSV glycoprotein 5 and the Toll-like Receptor-5 agonist Salmonella Typhimurium FljB
Abstract
Background: Porcine reproductive and respiratory syndrome (PRRS) is characterized by severe reproductive failure and severe pneumonia in neonatal pigs and is caused by PRRS virus (PRRSV). Glycoprotein 5 (GP5) from PRRSV is a key inducer of neutralizing antibodies. Flagellin, a toll-like receptor 5 (TLR-5) agonist, is an effective inducer of innate immune responses. This study presents a novel PRRSV vaccine candidate based on the adjuvant effect of Salmonella Typhimurium FljB fused with PRRSV GP5.
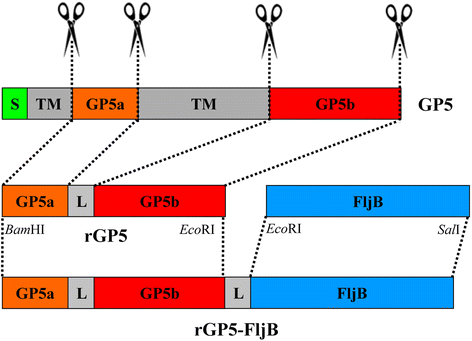
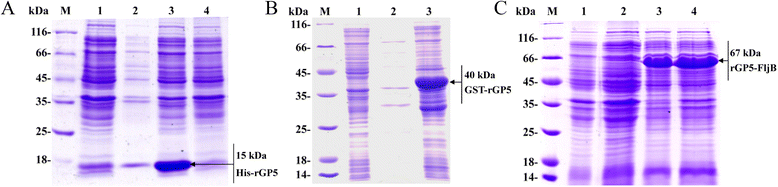
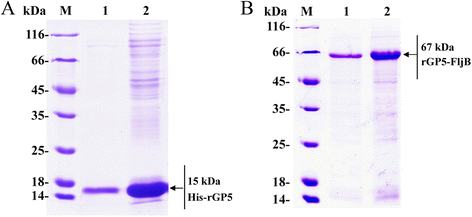
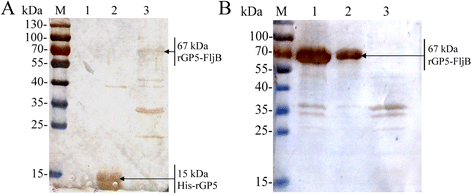
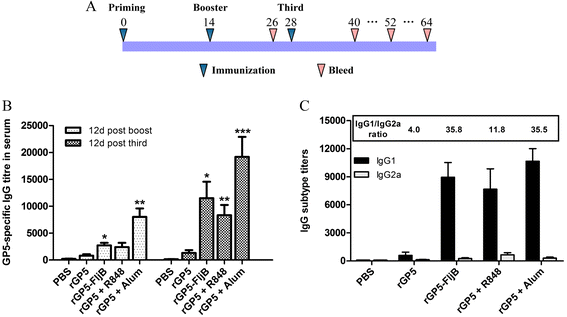
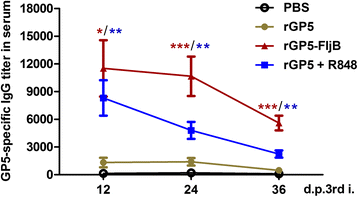
Results: A truncated rGP5 gene lacking the signal peptide and transmembrane sequences was amplified and inserted into prokaryotic expression vectors, pColdI or pGEX-6p-1. Salmonella Typhimurium flagellin fljB was amplified and inserted into the plasmid pCold-rGP5, generating recombinant plasmid pCold-rGP5-fljB. Histidine (His)-tagged rGP5 and fusion protein rGP5-FljB were induced with isopropyl-β-d-thiogalactoside, verified by SDS-PAGE and western blotting, and purified via Ni-NTA affinity columns. The TLR-5-specific bioactivity of fusion protein rGP5-FljB was determined by detecting the expression levels of the cytokine IL-8 in HEK293-mTLR5 cells by sandwich ELISA. The purified endotoxin-free proteins were administered intraperitoneally in a C3H/HeJ mouse model. The results show that immunization with the fusion protein rGP5-FljB induced a significantly enhanced GP5-specific and PRRSV-specific IgG response that persisted for almost 5 weeks. Co-administration of the rGP5 with R848 or Alum also yielded a higher IgG response than administration of rGP5 alone. The IgG1/IgG2a ratio in the rGP5-FljB immunization group was significantly higher (9-fold) than that in the rGP5 alone group and was equivalent to the response in the rGP5 + Alum immunization group, suggesting a strong Th2 immune response was induced by the fusion protein.
Conclusions: Purified fusion protein rGP5-FljB is capable of activating the innate immune response, as demonstrated by the results of our TLR-5-specific bioactivity assay, and FljB has adjuvant activity, as shown by the results from our administration of rGP5-FljB in a mouse model. Our findings confirm that FljB could serve as an excellent adjuvant for the production of GP5-specific and PRRSV-specific IgG antibodies as part of an induction of a robust humoral immune response.
Figures
Similar articles
-
A porcine reproductive and respiratory syndrome virus vaccine candidate based on PRRSV glycoprotein 5 and the Toll-like receptor 5 agonist Salmonella typhimurium flagellin.J Mol Microbiol Biotechnol. 2015;25(1):56-9. doi: 10.1159/000375496. J Mol Microbiol Biotechnol. 2015. PMID: 25766593
-
Recombinant adenovirus expressing GP5 and M fusion proteins of porcine reproductive and respiratory syndrome virus induce both humoral and cell-mediated immune responses in mice.Vet Immunol Immunopathol. 2006 Sep 15;113(1-2):169-80. doi: 10.1016/j.vetimm.2006.05.001. Epub 2006 Jun 13. Vet Immunol Immunopathol. 2006. PMID: 16777236
-
Immune response to Fc tagged GP5 glycoproteins of porcine reproductive and respiratory syndrome virus.Viral Immunol. 2014 Sep;27(7):343-9. doi: 10.1089/vim.2014.0041. Epub 2014 Jul 11. Viral Immunol. 2014. PMID: 25014350
-
Adjuvants for porcine reproductive and respiratory syndrome virus vaccines.Vet Immunol Immunopathol. 2009 May 15;129(1-2):1-13. doi: 10.1016/j.vetimm.2008.12.018. Epub 2008 Dec 11. Vet Immunol Immunopathol. 2009. PMID: 19157569 Review.
-
Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: Current status and future direction.Vaccine. 2015 Jun 17;33(27):3065-72. doi: 10.1016/j.vaccine.2015.04.102. Epub 2015 May 14. Vaccine. 2015. PMID: 25980425 Review.
Cited by
-
Development of a Ferritin Protein Nanoparticle Vaccine with PRRSV GP5 Protein.Viruses. 2024 Jun 20;16(6):991. doi: 10.3390/v16060991. Viruses. 2024. PMID: 38932282 Free PMC article.
-
Recombinant Live-Attenuated Salmonella Vaccine for Veterinary Use.Vaccines (Basel). 2024 Nov 26;12(12):1319. doi: 10.3390/vaccines12121319. Vaccines (Basel). 2024. PMID: 39771981 Free PMC article. Review.
-
Heterologous expression of porcine reproductive and respiratory syndrome virus glycoprotein 5 in Bordetella bronchisepticaaroA mutant.J Vet Med Sci. 2016 Nov 1;78(10):1625-1629. doi: 10.1292/jvms.15-0687. Epub 2016 Jun 24. J Vet Med Sci. 2016. PMID: 27349762 Free PMC article. Clinical Trial.
References
-
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142(3):629–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

