ATM facilitates mouse gammaherpesvirus reactivation from myeloid cells during chronic infection
- PMID: 26001649
- PMCID: PMC4516584
- DOI: 10.1016/j.virol.2015.04.026
ATM facilitates mouse gammaherpesvirus reactivation from myeloid cells during chronic infection
Abstract
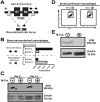
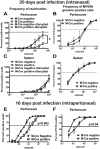
Gammaherpesviruses are cancer-associated pathogens that establish life-long infection in most adults. Insufficiency of Ataxia-Telangiectasia mutated (ATM) kinase leads to a poor control of chronic gammaherpesvirus infection via an unknown mechanism that likely involves a suboptimal antiviral response. In contrast to the phenotype in the intact host, ATM facilitates gammaherpesvirus reactivation and replication in vitro. We hypothesized that ATM mediates both pro- and antiviral activities to regulate chronic gammaherpesvirus infection in an immunocompetent host. To test the proposed proviral activity of ATM in vivo, we generated mice with ATM deficiency limited to myeloid cells. Myeloid-specific ATM deficiency attenuated gammaherpesvirus infection during the establishment of viral latency. The results of our study uncover a proviral role of ATM in the context of gammaherpesvirus infection in vivo and support a model where ATM combines pro- and antiviral functions to facilitate both gammaherpesvirus-specific T cell immune response and viral reactivation in vivo.
Keywords: ATM; Gammaherpesvirus; Myeloid cell; Reactivation; T cell response.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
B Cell-Specific Expression of Ataxia-Telangiectasia Mutated Protein Kinase Promotes Chronic Gammaherpesvirus Infection.J Virol. 2017 Sep 12;91(19):e01103-17. doi: 10.1128/JVI.01103-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28701397 Free PMC article.
-
Conserved Gammaherpesvirus Protein Kinase Counters the Antiviral Effects of Myeloid Cell-Specific STAT1 Expression To Promote the Establishment of Splenic B Cell Latency.J Virol. 2021 Aug 10;95(17):e0085921. doi: 10.1128/JVI.00859-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34132573 Free PMC article.
-
Interferon Regulatory Factor 3 Supports the Establishment of Chronic Gammaherpesvirus Infection in a Route- and Dose-Dependent Manner.J Virol. 2021 Apr 12;95(9):e02208-20. doi: 10.1128/JVI.02208-20. Print 2021 Apr 12. J Virol. 2021. PMID: 33597211 Free PMC article.
-
Gamma interferon blocks gammaherpesvirus reactivation from latency in a cell type-specific manner.J Virol. 2007 Jun;81(11):6134-40. doi: 10.1128/JVI.00108-07. Epub 2007 Mar 14. J Virol. 2007. PMID: 17360749 Free PMC article. Review.
-
Pathogenesis and host control of gammaherpesviruses: lessons from the mouse.Annu Rev Immunol. 2011;29:351-97. doi: 10.1146/annurev-immunol-072710-081639. Annu Rev Immunol. 2011. PMID: 21219186 Review.
Cited by
-
B Cell-Intrinsic Expression of Interferon Regulatory Factor 1 Supports Chronic Murine Gammaherpesvirus 68 Infection.J Virol. 2020 Jun 16;94(13):e00399-20. doi: 10.1128/JVI.00399-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32321819 Free PMC article.
-
Conserved Gammaherpesvirus Protein Kinase Selectively Promotes Irrelevant B Cell Responses.J Virol. 2019 Apr 3;93(8):e01760-18. doi: 10.1128/JVI.01760-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30728267 Free PMC article.
-
LXR Alpha Restricts Gammaherpesvirus Reactivation from Latently Infected Peritoneal Cells.J Virol. 2019 Mar 5;93(6):e02071-18. doi: 10.1128/JVI.02071-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30602604 Free PMC article.
-
Conquering the Host: Determinants of Pathogenesis Learned from Murine Gammaherpesvirus 68.Annu Rev Virol. 2021 Sep 29;8(1):349-371. doi: 10.1146/annurev-virology-011921-082615. Annu Rev Virol. 2021. PMID: 34586873 Free PMC article.
-
Murine Gammaherpesvirus 68 LANA and SOX Homologs Counteract ATM-Driven p53 Activity during Lytic Viral Replication.J Virol. 2015 Dec 16;90(5):2571-85. doi: 10.1128/JVI.02867-15. J Virol. 2015. PMID: 26676792 Free PMC article.
References
-
- Ben-Zvi A, Soffer D, Yatziv S. Disseminated Herpes simplex virus infection in ataxia-telangiectasia. Acta Paediatr Scand. 1978;67:667–670. - PubMed
-
- Campbell TB, Borok M, Gwanzura L, MaWhinney S, White IE, Ndemera B, Gudza I, Fitzpatrick L, Schooley RT. Relationship of human herpesvirus 8 peripheral blood virus load and Kaposi's sarcoma clinical stage. AIDS. 2000;14:2109–2116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

