Elucidation of changes in molecular signalling leading to increased cellular transformation in oncogenically progressed human bronchial epithelial cells exposed to radiations of increasing LET
- PMID: 26001755
- PMCID: PMC4635632
- DOI: 10.1093/mutage/gev028
Elucidation of changes in molecular signalling leading to increased cellular transformation in oncogenically progressed human bronchial epithelial cells exposed to radiations of increasing LET
Abstract
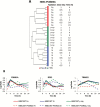
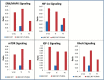
The early transcriptional response and subsequent induction of anchorage-independent growth after exposure to particles of high Z and energy (HZE) as well as γ-rays were examined in human bronchial epithelial cells (HBEC3KT) immortalised without viral oncogenes and an isogenic variant cell line whose p53 expression was suppressed but that expressed an active mutant K-RAS(V12) (HBEC3KT-P53KRAS). Cell survival following irradiation showed that HBEC3KT-P53KRAS cells were more radioresistant than HBEC3KT cells irrespective of the radiation species. In addition, radiation enhanced the ability of the surviving HBEC3KT-P53RAS cells but not the surviving HBEC3KT cells to grow in anchorage-independent fashion (soft agar colony formation). HZE particle irradiation was far more efficient than γ-rays at rendering HBEC3KT-P53RAS cells permissive for soft agar growth. Gene expression profiles after radiation showed that the molecular response to radiation for HBEC3KT-P53RAS, similar to that for HBEC3KT cells, varies with radiation quality. Several pathways associated with anchorage independent growth, including the HIF-1α, mTOR, IGF-1, RhoA and ERK/MAPK pathways, were over-represented in the irradiated HBEC3KT-P53RAS cells compared to parental HBEC3KT cells. These results suggest that oncogenically progressed human lung epithelial cells are at greater risk for cellular transformation and carcinogenic risk after ionising radiation, but particularly so after HZE radiations. These results have implication for: (i) terrestrial radiation and suggests the possibility of enhanced carcinogenic risk from diagnostic CT screens used for early lung cancer detection; (ii) enhanced carcinogenic risk from heavy particles used in radiotherapy; and (iii) for space radiation, raising the possibility that astronauts harbouring epithelial regions of dysplasia or hyperplasia within the lung that contain oncogenic changes, may have a greater risk for lung cancers based upon their exposure to heavy particles present in the deep space environment.
© The Author 2015. Published by Oxford University Press on behalf of the UK Environmental Mutagen Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Distinct transcriptome profiles identified in normal human bronchial epithelial cells after exposure to γ-rays and different elemental particles of high Z and energy.BMC Genomics. 2013 Jun 1;14:372. doi: 10.1186/1471-2164-14-372. BMC Genomics. 2013. PMID: 23724988 Free PMC article.
-
A single low dose of Fe ions can cause long-term biological responses in NL20 human bronchial epithelial cells.Radiat Environ Biophys. 2018 Mar;57(1):31-40. doi: 10.1007/s00411-017-0719-0. Epub 2017 Nov 10. Radiat Environ Biophys. 2018. PMID: 29127482
-
Multiple oncogenic changes (K-RAS(V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells.Cancer Res. 2006 Feb 15;66(4):2116-28. doi: 10.1158/0008-5472.CAN-05-2521. Cancer Res. 2006. PMID: 16489012
-
Radiogenic cell transformation and carcinogenesis.ASGSB Bull. 1995 Oct;8(2):106-12. ASGSB Bull. 1995. PMID: 11538546 Review.
-
Review of relative biological effectiveness dependence on linear energy transfer for low-LET radiations.J Radiol Prot. 2009 Mar;29(1):5-21. doi: 10.1088/0952-4746/29/1/R01. Epub 2009 Feb 18. J Radiol Prot. 2009. PMID: 19225189 Review.
Cited by
-
Gene Expression Studies for the Development of Particle Therapy.Int J Part Ther. 2018 Summer;5(1):49-59. doi: 10.14338/IJPT-18-00010.1. Epub 2018 Sep 21. Int J Part Ther. 2018. PMID: 30555854 Free PMC article.
-
Organotypic culture in three dimensions prevents radiation-induced transformation in human lung epithelial cells.Sci Rep. 2016 Aug 19;6:31669. doi: 10.1038/srep31669. Sci Rep. 2016. PMID: 27539227 Free PMC article.
-
Replication stress and FOXM1 drive radiation induced genomic instability and cell transformation.PLoS One. 2020 Nov 30;15(11):e0235998. doi: 10.1371/journal.pone.0235998. eCollection 2020. PLoS One. 2020. PMID: 33253193 Free PMC article.
-
Evaluating biomarkers to model cancer risk post cosmic ray exposure.Life Sci Space Res (Amst). 2016 Jun;9:19-47. doi: 10.1016/j.lssr.2016.05.004. Epub 2016 May 21. Life Sci Space Res (Amst). 2016. PMID: 27345199 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

