Impairment of cognitive function by chemotherapy: association with the disruption of phase-locking and synchronization in anterior cingulate cortex
- PMID: 26001812
- PMCID: PMC4490721
- DOI: 10.1186/s13041-015-0125-y
Impairment of cognitive function by chemotherapy: association with the disruption of phase-locking and synchronization in anterior cingulate cortex
Abstract
Background: Patients following prolonged cancer chemotherapy are at high risk of emotional and cognitive deficits. Research indicates that the brain neuronal temporal coding and synaptic long-term potentiation (LTP) are critical in memory and perception. We studied the effects of cisplatin on induction of LTP in the basolateral amygdala (BLA)-anterior cingulate cortex (ACC) pathway, characterized the coordination of spike timing with local theta oscillation, and identified synchrony in the BLA-ACC network integrity.
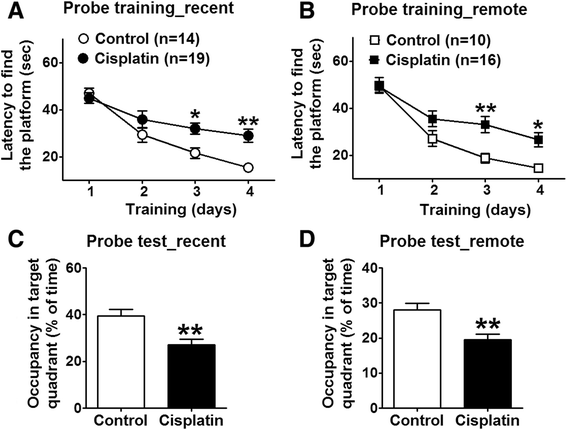
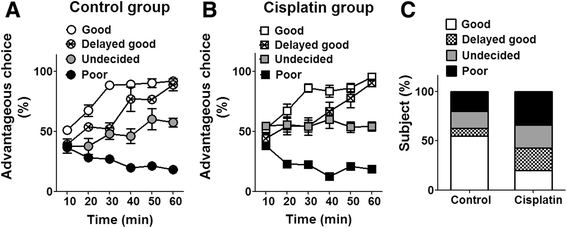
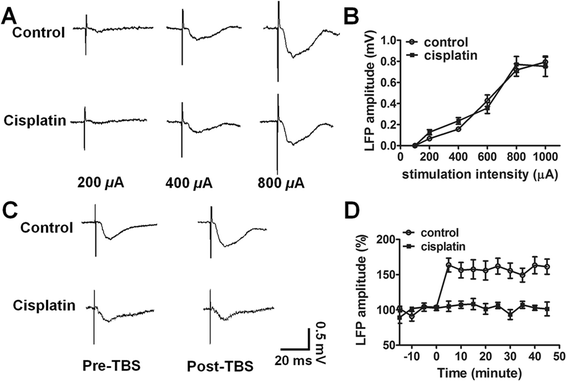
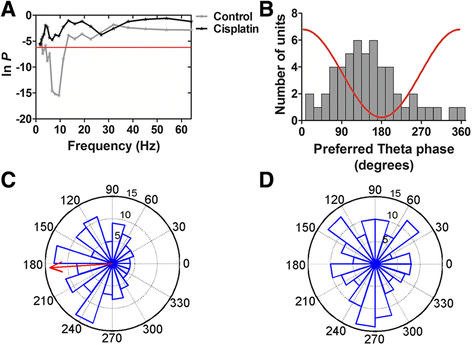
Results: In the study presented, the impacts of cisplatin on emotional and cognitive functions were investigated by elevated plus-maze test, Morris water maze test, and rat Iowa gambling task (RGT). Electrophysiological recordings were conducted to study long-term potentiation. Simultaneous recordings from multi-electrodes were performed to characterize the neural spike firing and ongoing theta oscillation of local field potential (LFP), and to clarify the synchronization of large scale of theta oscillation in the BLA-ACC pathway. Cisplatin-treated rats demonstrated anxiety- like behavior, exhibited impaired spatial reference memory. RGT showed decrease of the percentage of good decision-makers, and increase in the percentage of maladaptive behavior (delay-good decision-makers plus poor decision-makers). Cisplatin suppressed the LTP, and disrupted the phase-locking of ACC single neural firings to the ongoing theta oscillation; further, cisplatin interrupted the synchrony in the BLA-ACC pathway.
Conclusions: We provide the first direct evidence that the cisplatin interrupts theta-frequency phase-locking of ACC neurons. The block of LTP and disruption of synchronized theta oscillations in the BLA-ACC pathway are associated with emotional and cognitive deficits in rats, following cancer chemotherapy.
Figures



Similar articles
-
Impairment of decision making and disruption of synchrony between basolateral amygdala and anterior cingulate cortex in the maternally separated rat.Neurobiol Learn Mem. 2016 Dec;136:74-85. doi: 10.1016/j.nlm.2016.09.015. Epub 2016 Sep 21. Neurobiol Learn Mem. 2016. PMID: 27664716
-
Decision-making deficits associated with disrupted synchronization between basolateral amygdala and anterior cingulate cortex in rats after tooth loss.Prog Neuropsychopharmacol Biol Psychiatry. 2015 Jul 3;60:26-35. doi: 10.1016/j.pnpbp.2015.02.002. Epub 2015 Feb 12. Prog Neuropsychopharmacol Biol Psychiatry. 2015. PMID: 25684327
-
Synaptic Plasticity and Synchrony in the Anterior Cingulate Cortex Circuitry: A Neural Network Approach to Causality of Chronic Visceral Pain and Associated Cognitive Deficits.Adv Neurobiol. 2018;21:219-245. doi: 10.1007/978-3-319-94593-4_8. Adv Neurobiol. 2018. PMID: 30334224
-
Memory formation by neuronal synchronization.Brain Res Rev. 2006 Aug 30;52(1):170-82. doi: 10.1016/j.brainresrev.2006.01.007. Epub 2006 Mar 20. Brain Res Rev. 2006. PMID: 16545463 Review.
-
Neural Mechanisms Underlying Anxiety-Chronic Pain Interactions.Trends Neurosci. 2016 Mar;39(3):136-145. doi: 10.1016/j.tins.2016.01.006. Epub 2016 Feb 12. Trends Neurosci. 2016. PMID: 26878750 Review.
Cited by
-
Suppression of Pain in the Late Phase of Chronic Trigeminal Neuropathic Pain Failed to Rescue the Decision-Making Deficits in Rats.Int J Mol Sci. 2021 Jul 22;22(15):7846. doi: 10.3390/ijms22157846. Int J Mol Sci. 2021. PMID: 34360612 Free PMC article.
-
Abnormal circadian oscillation of hippocampal MAPK activity and power spectrums in NF1 mutant mice.Mol Brain. 2017 Jul 3;10(1):29. doi: 10.1186/s13041-017-0309-8. Mol Brain. 2017. PMID: 28673309 Free PMC article.
-
Poor Decision Making and Sociability Impairment Following Central Serotonin Reduction in Inducible TPH2-Knockdown Rats.Int J Mol Sci. 2024 May 3;25(9):5003. doi: 10.3390/ijms25095003. Int J Mol Sci. 2024. PMID: 38732220 Free PMC article.
-
Chronic ciguatoxin poisoning causes emotional and cognitive dysfunctions in rats.Toxicol Res (Camb). 2016 Jun 8;6(2):179-187. doi: 10.1039/c5tx00475f. eCollection 2017 Mar 1. Toxicol Res (Camb). 2016. PMID: 30090488 Free PMC article.
-
Chemogenetic Activation of Astrocytes in the Basolateral Amygdala Contributes to Fear Memory Formation by Modulating the Amygdala-Prefrontal Cortex Communication.Int J Mol Sci. 2022 May 29;23(11):6092. doi: 10.3390/ijms23116092. Int J Mol Sci. 2022. PMID: 35682767 Free PMC article.
References
-
- Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2008;19:623–9. doi: 10.1093/annonc/mdm500. - DOI - PubMed
-
- Jacobs S, McCully CL, Murphy RF, Bacher J, Balis FM, Fox E. Extracellular fluid concentrations of cisplatin, carboplatin, and oxaliplatin in brain, muscle, and blood measured using microdialysis in nonhuman primates. Cancer Chemother Pharmacol. 2010;65:817–24. doi: 10.1007/s00280-009-1085-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

