Chemokines and microRNAs in atherosclerosis
- PMID: 26001902
- PMCID: PMC4531138
- DOI: 10.1007/s00018-015-1925-z
Chemokines and microRNAs in atherosclerosis
Abstract
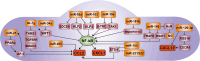
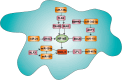
The crucial role of chemokines in the initiation and progression of atherosclerosis has been widely recognized. Through essential functions in leukocyte recruitment, chemokines govern the infiltration with mononuclear cells and macrophage accumulation in atherosclerotic lesions. Beyond recruitment, chemokines also provide homeostatic functions supporting cell survival and mediating the mobilization and homing of progenitor cells. As a new regulatory layer, several microRNAs (miRNAs) have been found to modulate the function of endothelial cells (ECs), smooth muscle cells and macrophages by controlling the expression levels of chemokines and thereby affecting different stages in the progression of atherosclerosis. For instance, the expression of CXCL1 can be down-regulated by miR-181b, which inhibits nuclear factor-κB activation in atherosclerotic endothelium, thus attenuating the adhesive properties of ECs and exerting early atheroprotective effects. Conversely, CXCL12 expression can be induced by miR-126 in ECs through an auto-amplifying feedback loop to facilitate endothelial regeneration, thus limiting atherosclerosis and mediating plaque stabilization. In contrast, miR-155 plays a pro-atherogenic role by promoting the expression of CCL2 in M1-type macrophages, thereby enhancing vascular inflammation. Herein, we will review novel aspects of chemokines and their regulation by miRNAs during atherogenesis. Understanding the complex cross-talk of miRNAs controlling chemokine expression may open novel therapeutic options to treat atherosclerosis.
Conflict of interest statement
This study has no disclosures.
Figures
References
-
- Zernecke A, Weber C. Chemokines in atherosclerosis: proceedings resumed. Arterioscler Thromb Vasc Biol. 2014;34(4):742–750. - PubMed
-
- Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. - PubMed
-
- Nazari-Jahantigh M, Egea V, Schober A, Weber C. MicroRNA-specific regulatory mechanisms in atherosclerosis. J Mol Cell Cardiol. 2014 - PubMed
-
- Wei Y, Nazari-Jahantigh M, Neth P, Weber C, Schober A. MicroRNA-126, -145, and -155: a therapeutic triad in atherosclerosis? Arterioscler Thromb Vasc Biol. 2013;33(3):449–454. - PubMed
-
- Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH, Locati M, Luster AD, Mantovani A, Matsushima K, Murphy PM, Nibbs R, Nomiyama H, Power CA, Proudfoot AE, Rosenkilde MM, Rot A, Sozzani S, Thelen M, Yoshie O, Zlotnik A. International Union of Basic and Clinical Pharmacology [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014;66(1):1–79. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

