Cytoplasmic dynein and early endosome transport
- PMID: 26001903
- PMCID: PMC4534323
- DOI: 10.1007/s00018-015-1926-y
Cytoplasmic dynein and early endosome transport
Abstract
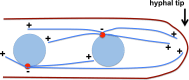
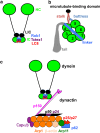
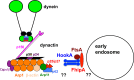
Microtubule-based distribution of organelles/vesicles is crucial for the function of many types of eukaryotic cells and the molecular motor cytoplasmic dynein is required for transporting a variety of cellular cargos toward the microtubule minus ends. Early endosomes represent a major cargo of dynein in filamentous fungi, and dynein regulators such as LIS1 and the dynactin complex are both required for early endosome movement. In fungal hyphae, kinesin-3 and dynein drive bi-directional movements of early endosomes. Dynein accumulates at microtubule plus ends; this accumulation depends on kinesin-1 and dynactin, and it is important for early endosome movements towards the microtubule minus ends. The physical interaction between dynein and early endosome requires the dynactin complex, and in particular, its p25 component. The FTS-Hook-FHIP (FHF) complex links dynein-dynactin to early endosomes, and within the FHF complex, Hook interacts with dynein-dynactin, and Hook-early endosome interaction depends on FHIP and FTS.
Figures

References
-
- Engqvist-Goldstein AE, Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol. 2003;19:287–332. - PubMed
-
- Mooren OL, Galletta BJ, Cooper JA. Roles for actin assembly in endocytosis. Annu Rev Biochem. 2012;81:661–686. - PubMed
-
- Peñalva MA. Endocytosis in filamentous fungi: Cinderella gets her reward. Curr Opin Microbiol. 2010;13:684–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

