Na+/Taurocholate Cotransporting Polypeptide and Apical Sodium-Dependent Bile Acid Transporter Are Involved in the Disposition of Perfluoroalkyl Sulfonates in Humans and Rats
- PMID: 26001962
- PMCID: PMC4607751
- DOI: 10.1093/toxsci/kfv102
Na+/Taurocholate Cotransporting Polypeptide and Apical Sodium-Dependent Bile Acid Transporter Are Involved in the Disposition of Perfluoroalkyl Sulfonates in Humans and Rats
Abstract
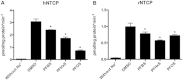
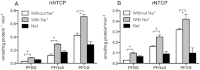
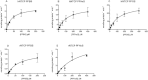
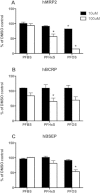
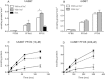
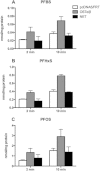
Among the perfluoroalkyl sulfonates (PFASs), perfluorohexane sulfonate (PFHxS), and perfluorooctane sulfonate (PFOS) have half-lives of several years in humans, mainly due to slow renal clearance and potential hepatic accumulation. Both compounds undergo enterohepatic circulation. To determine whether transporters involved in the enterohepatic circulation of bile acids are also involved in the disposition of PFASs, uptake of perfluorobutane sulfonate (PFBS), PFHxS, and PFOS was measured using freshly isolated human and rat hepatocytes in the absence or presence of sodium. The results demonstrated sodium-dependent uptake for all 3 PFASs. Given that the Na(+)/taurocholate cotransporting polypeptide (NTCP) and the apical sodium-dependent bile salt transporter (ASBT) are essential for the enterohepatic circulation of bile acids, transport of PFASs was investigated in stable CHO Flp-In cells for human NTCP or HEK293 cells transiently expressing rat NTCP, human ASBT, and rat ASBT. The results demonstrated that both human and rat NTCP can transport PFBS, PFHxS, and PFOS. Kinetics with human NTCP revealed Km values of 39.6, 112, and 130 µM for PFBS, PFHxS, and PFOS, respectively. For rat NTCP Km values were 76.2 and 294 µM for PFBS and PFHxS, respectively. Only PFOS was transported by human ASBT whereas rat ASBT did not transport any of the tested PFASs. Human OSTα/β was also able to transport all 3 PFASs. In conclusion, these results suggest that the long half-live and the hepatic accumulation of PFOS in humans are at least, in part, due to transport by NTCP and ASBT.
Keywords: hepatocytes; perfluoroalkyl sulfonates; perfluorobutane sulfonate; perfluorohexane sulfonate; perfluorooctane sulfonate.
© The Author 2015. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Organic Anion Transporting Polypeptides Contribute to the Disposition of Perfluoroalkyl Acids in Humans and Rats.Toxicol Sci. 2017 Mar 1;156(1):84-95. doi: 10.1093/toxsci/kfw236. Toxicol Sci. 2017. PMID: 28013215 Free PMC article.
-
The sodium bile salt cotransport family SLC10.Pflugers Arch. 2004 Feb;447(5):566-70. doi: 10.1007/s00424-003-1130-z. Epub 2003 Jul 8. Pflugers Arch. 2004. PMID: 12851823 Review.
-
Uptake of ursodeoxycholate and its conjugates by human hepatocytes: role of Na(+)-taurocholate cotransporting polypeptide (NTCP), organic anion transporting polypeptide (OATP) 1B1 (OATP-C), and oatp1B3 (OATP8).Mol Pharm. 2006 Jan-Feb;3(1):70-7. doi: 10.1021/mp050063u. Mol Pharm. 2006. PMID: 16686371
-
Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter.J Clin Invest. 1997 Dec 1;100(11):2714-21. doi: 10.1172/JCI119816. J Clin Invest. 1997. PMID: 9389734 Free PMC article.
-
The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships.Naunyn Schmiedebergs Arch Pharmacol. 2006 Mar;372(6):413-31. doi: 10.1007/s00210-006-0043-8. Epub 2006 Mar 16. Naunyn Schmiedebergs Arch Pharmacol. 2006. PMID: 16541252 Review.
Cited by
-
Free Cholesterol Affects the Function and Localization of Human Na+/Taurocholate Cotransporting Polypeptide (NTCP) and Organic Cation Transporter 1 (OCT1).Int J Mol Sci. 2022 Jul 30;23(15):8457. doi: 10.3390/ijms23158457. Int J Mol Sci. 2022. PMID: 35955590 Free PMC article.
-
A clinically relevant polymorphism in the Na+/taurocholate cotransporting polypeptide (NTCP) occurs at a rheostat position.J Biol Chem. 2021 Jan-Jun;296:100047. doi: 10.1074/jbc.RA120.014889. Epub 2020 Dec 2. J Biol Chem. 2021. PMID: 33168628 Free PMC article.
-
Exposure of Mice to a PFAS Mixture and Outcomes Related to Circulating Lipids, Bile Acid Excretion, and the Intestinal Transporter ASBT.Environ Health Perspect. 2024 Aug;132(8):87007. doi: 10.1289/EHP14339. Epub 2024 Aug 23. Environ Health Perspect. 2024. PMID: 39177951 Free PMC article.
-
Why is elevation of serum cholesterol associated with exposure to perfluoroalkyl substances (PFAS) in humans? A workshop report on potential mechanisms.Toxicology. 2021 Jul;459:152845. doi: 10.1016/j.tox.2021.152845. Epub 2021 Jul 8. Toxicology. 2021. PMID: 34246716 Free PMC article. Review.
-
Simultaneous determination of perfluoroalkyl substances and bile acids in human serum using ultra-high-performance liquid chromatography-tandem mass spectrometry.Anal Bioanal Chem. 2020 Apr;412(10):2251-2259. doi: 10.1007/s00216-019-02263-6. Epub 2019 Nov 23. Anal Bioanal Chem. 2020. PMID: 31760452 Free PMC article.
References
-
- Andersen M. E., Butenhoff J. L., Chang S. C., Farrar D. G., Kennedy G. L., Jr, Lau C., Olsen G. W., Seed J., Wallace K. B. (2008). Perfluoroalkyl acids and related chemistries–toxicokinetics and modes of action. Toxicol. Sci. 102, 3–14. - PubMed
-
- Ballatori N., Christian W. V., Lee J. Y., Dawson P. A., Soroka C. J., Boyer J. L., Madejczyk M. S., Li N. (2005). OSTα-OSTβ: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 42, 1270–1279. - PubMed
-
- Benedetti A., Di Sario A., Marucci L., Svegliati-Baroni G., Schteingart C. D., Ton-Nu H. T., Hofmann A. F. (1997). Carrier-mediated transport of conjugated bile acids across the basolateral membrane of biliary epithelial cells. Am. J. Physiol. 272, G1416–G1424. - PubMed
-
- Bi Y. A., Qiu X., Rotter C. J., Kimoto E., Piotrowski M., Varma M. V., Ei-Kattan A. F., Lai Y. (2013). Quantitative assessment of the contribution of sodium-dependent taurocholate co-transporting polypeptide (NTCP) to the hepatic uptake of rosuvastatin, pitavastatin and fluvastatin. Biopharm. Drug Dispos. 34, 452–461. - PubMed
-
- Buck R. C., Franklin J., Berger U., Conder J. M., Cousins I. T., de Voogt P., Jensen A. A., Kannan K., Mabury S. A., van Leeuwen S. P. (2011). Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag. 7, 513–541. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

