The intestinal microbiome in spondyloarthritis
- PMID: 26002022
- PMCID: PMC4489849
- DOI: 10.1097/BOR.0000000000000187
The intestinal microbiome in spondyloarthritis
Abstract
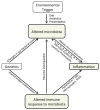
Purpose of review: Microbial dysbiosis in the gut is emerging as a common component in various inflammatory disorders including spondyloarthritis (SpA). The depth of this influence has begun to be realized with next-generation sequencing of the gut microbiome providing unbiased assessment of previously uncharted bacterial populations.
Recent findings: Decreased numbers of Firmicutes, a major phyla of gut commensals, especially the species Faecalibacterium prausnitzii and Clostridium leptum have been found in various inflammatory disorders including SpA and inflammatory bowel disease (IBD), and could be an important link between SpA and gut inflammation. Multiple studies in ankylosing spondylitis, psoriatic arthritis, juvenile SpA, and animal models of SpA are revealing common bacterial associations among these diseases as well as IBD.
Summary: We are beginning to appreciate the complex relationship between the gut microbiome and host immune regulation and dysregulation in health and disease. Potentially important differences have been revealed in SpA, but cause and effect relationships remain far from established. Many critical questions remain to be answered before we can apply new knowledge to improve therapeutics in SpA.
Conflict of interest statement
The authors declare no conflicts of interest relevant to this work.
Figures
References
-
- Taurog JD, Maika SD, Satumtira N, Dorris ML, McLean IL, Yanagisawa H, Sayad A, Stagg AJ, Fox GM, Le O’Brien A, et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169:209–223. - PubMed
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

