Application of Absorption Modeling in Rational Design of Drug Product Under Quality-by-Design Paradigm
- PMID: 26002509
- PMCID: PMC4540722
- DOI: 10.1208/s12248-015-9781-1
Application of Absorption Modeling in Rational Design of Drug Product Under Quality-by-Design Paradigm
Abstract
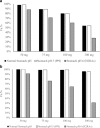
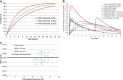
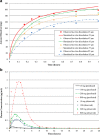
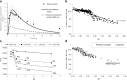
Physiologically based absorption models can be an important tool in understanding product performance and hence implementation of Quality by Design (QbD) in drug product development. In this report, we show several case studies to demonstrate the potential application of absorption modeling in rational design of drug product under the QbD paradigm. The examples include application of absorption modeling—(1) prior to first-in-human studies to guide development of a formulation with minimal sensitivity to higher gastric pH and hence reduced interaction when co-administered with PPIs and/or H2RAs, (2) design of a controlled release formulation with optimal release rate to meet trough plasma concentrations and enable QD dosing, (3) understanding the impact of API particle size distribution on tablet bioavailability and guide formulation design in late-stage development, (4) assess impact of API phase change on product performance to guide specification setting, and (5) investigate the effect of dissolution rate changes on formulation bioperformance and enable appropriate specification setting. These case studies are meant to highlight the utility of physiologically based absorption modeling in gaining a thorough understanding of the product performance and the critical factors impacting performance to drive design of a robust drug product that would deliver the optimal benefit to the patients.
Figures
Similar articles
-
Physiologically Based Absorption Modeling for Amorphous Solid Dispersion Formulations.Mol Pharm. 2016 Sep 6;13(9):3206-15. doi: 10.1021/acs.molpharmaceut.6b00424. Epub 2016 Jul 29. Mol Pharm. 2016. PMID: 27442959
-
In silico predictions of gastrointestinal drug absorption in pharmaceutical product development: application of the mechanistic absorption model GI-Sim.Eur J Pharm Sci. 2013 Jul 16;49(4):679-98. doi: 10.1016/j.ejps.2013.05.019. Epub 2013 May 29. Eur J Pharm Sci. 2013. PMID: 23727464
-
In vitro dissolution methodology, mini-Gastrointestinal Simulator (mGIS), predicts better in vivo dissolution of a weak base drug, dasatinib.Eur J Pharm Sci. 2015 Aug 30;76:203-12. doi: 10.1016/j.ejps.2015.05.013. Epub 2015 May 12. Eur J Pharm Sci. 2015. PMID: 25978875
-
Understanding the effect of API properties on bioavailability through absorption modeling.AAPS J. 2008 Dec;10(4):516-25. doi: 10.1208/s12248-008-9061-4. Epub 2008 Nov 6. AAPS J. 2008. PMID: 19002590 Free PMC article. Review.
-
Oral biopharmaceutics tools - time for a new initiative - an introduction to the IMI project OrBiTo.Eur J Pharm Sci. 2014 Jun 16;57:292-9. doi: 10.1016/j.ejps.2013.10.012. Epub 2013 Nov 1. Eur J Pharm Sci. 2014. PMID: 24189462 Review.
Cited by
-
The growing role of precision and personalized medicine for cancer treatment.Technology (Singap World Sci). 2018 Sep-Dec;6(3-4):79-100. doi: 10.1142/S2339547818300020. Epub 2019 Jan 11. Technology (Singap World Sci). 2018. PMID: 30713991 Free PMC article.
-
Developing a Formulation Strategy Coupled with PBPK Modeling and Simulation for the Weakly Basic Drug Albendazole.Pharmaceutics. 2023 Mar 23;15(4):1040. doi: 10.3390/pharmaceutics15041040. Pharmaceutics. 2023. PMID: 37111526 Free PMC article.
-
A Bayesian population physiologically based pharmacokinetic absorption modeling approach to support generic drug development: application to bupropion hydrochloride oral dosage forms.J Pharmacokinet Pharmacodyn. 2021 Dec;48(6):893-908. doi: 10.1007/s10928-021-09778-5. Epub 2021 Sep 22. J Pharmacokinet Pharmacodyn. 2021. PMID: 34553275 Free PMC article.
-
Justification of disintegration testing beyond current FDA criteria using in vitro and in silico models.Drug Des Devel Ther. 2017 Apr 11;11:1163-1174. doi: 10.2147/DDDT.S131213. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28442890 Free PMC article.
-
Physiologically Based Pharmacokinetic Modeling of Extracellular Vesicles.Biology (Basel). 2023 Aug 29;12(9):1178. doi: 10.3390/biology12091178. Biology (Basel). 2023. PMID: 37759578 Free PMC article. Review.
References
-
- U.S. Department of Health and Human Services, Food and Drug Administration, Guidance for Industry: Q8(R2) Pharmaceutical development. 2009.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

