Bisecting Galactose as a Feature of N-Glycans of Wild-type and Mutant Caenorhabditis elegans
- PMID: 26002521
- PMCID: PMC4523199
- DOI: 10.1074/mcp.M115.049817
Bisecting Galactose as a Feature of N-Glycans of Wild-type and Mutant Caenorhabditis elegans
Abstract
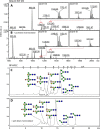
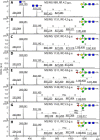
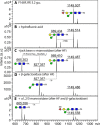
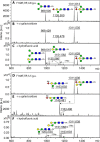
The N-glycosylation of the model nematode Caenorhabditis elegans has proven to be highly variable and rather complex; it is an example to contradict the existing impression that "simple" organisms possess also a rather simple glycomic capacity. In previous studies in a number of laboratories, N-glycans with up to four fucose residues have been detected. However, although the linkage of three fucose residues to the N,N'-diacetylchitobiosyl core has been proven by structural and enzymatic analyses, the nature of the fourth fucose has remained uncertain. By constructing a triple mutant with deletions in the three genes responsible for core fucosylation (fut-1, fut-6 and fut-8), we have produced a nematode strain lacking products of these enzymes, but still retaining maximally one fucose residue on its N-glycans. Using mass spectrometry and HPLC in conjunction with chemical and enzymatic treatments as well as NMR, we examined a set of α-mannosidase-resistant N-glycans. Within this glycomic subpool, we can reveal that the core β-mannose can be trisubstituted and so carries not only the ubiquitous α1,3- and α1,6-mannose residues, but also a "bisecting" β-galactose, which is substoichiometrically modified with fucose or methylfucose. In addition, the α1,3-mannose can also be α-galactosylated. Our data, showing the presence of novel N-glycan modifications, will enable more targeted studies to understand the biological functions and interactions of nematode glycans.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Sommer R. J., Streit A. (2011) Comparative genetics and genomics of nematodes: genome structure, development, and lifestyle. Annu. Rev. Genet. 45, 1–20 - PubMed
-
- Paschinger K., Gutternigg M., Rendić D., Wilson I. B. H. (2008) The N-glycosylation pattern of Caenorhabditis elegans. Carbohydr. Res. 343, 2041–2049 - PubMed
-
- Schachter H. (2009) Paucimannose N-glycans in Caenorhabditis elegans and Drosophila melanogaster. Carbohydr. Res. 344, 1391–1396 - PubMed
-
- Yazdanbakhsh M., Kremsner P. G., van Ree R. (2002) Allergy, parasites, and the hygiene hypothesis. Science 296, 490–494 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

