Tracking sub-clonal TP53 mutated tumor cells in human metastatic renal cell carcinoma
- PMID: 26002555
- PMCID: PMC4662490
- DOI: 10.18632/oncotarget.4220
Tracking sub-clonal TP53 mutated tumor cells in human metastatic renal cell carcinoma
Abstract
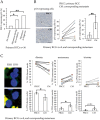
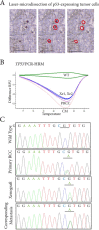
Renal Cell Carcinomas (RCCs) are heterogeneous tumors with late acquisition of TP53 abnormalities during their evolution. They harbor TP53 abnormalities in their metastases. We aimed to study TP53 gene alterations in tissue samples from primary and metastatic RCCs in 36 patients followed up over a median of 4.2 years, and in xenografted issued from primary RCCs. In 36 primary RCCs systematically xenografted in mice, and in biopsies of metastases performed whenever possible during patient follow-up, we studied p53-expressing tumor cells and TP53 gene abnormalities.We identified TP53 gene alterations in primary tumors, metastases and xenografts. Quantification of tumors cells with TP53 gene alterations showed a significant increase in the metastases compared to the primary RCCs, and, strikingly, the xenografts were similar to the metastases and not to the primary RCCs from which they were derived.Using laser-microdissection of p53-expressing tumor cells, we identified TP53-mutated tumor cells in the xenografts derived from the primary RCC, and in a lung metastasis later developed in one patient. The mutation enabled us to track back their origin to a minority sub-clone in the primary heterogeneous RCC. Combining in situ and molecular analyses, we demonstrated a clonal expansion in a living patient with metastatic RCC.
Keywords: TP53 mutation; human RCC; metastases; sub-clonal tumor cells; tumor heterogeneity.
Conflict of interest statement
The authors declare no conflict of interest
Figures

Similar articles
-
CNPY2 promoted the proliferation of renal cell carcinoma cells and increased the expression of TP53.Biochem Biophys Res Commun. 2017 Apr 1;485(2):267-271. doi: 10.1016/j.bbrc.2017.02.095. Epub 2017 Feb 21. Biochem Biophys Res Commun. 2017. PMID: 28235487
-
High fidelity of driver chromosomal alterations among primary and metastatic renal cell carcinomas: implications for tumor clonal evolution and treatment.Mod Pathol. 2016 Nov;29(11):1347-1357. doi: 10.1038/modpathol.2016.133. Epub 2016 Jul 29. Mod Pathol. 2016. PMID: 27469331
-
Reprofiling Metastatic Samples for Chromosome 9p and 14q Aberrations as a Strategy to Overcome Tumor Heterogeneity in Clear-cell Renal Cell Carcinoma.Appl Immunohistochem Mol Morphol. 2017 Jan;25(1):39-43. doi: 10.1097/PAI.0000000000000257. Appl Immunohistochem Mol Morphol. 2017. PMID: 26509904
-
Are primary renal cell carcinoma and metastases of renal cell carcinoma the same cancer?Urol Oncol. 2016 May;34(5):215-20. doi: 10.1016/j.urolonc.2015.12.013. Epub 2016 Feb 2. Urol Oncol. 2016. PMID: 26850779 Review.
-
Neuroblastoma-associated Renal Cell Carcinoma: A Case Report and Review of the Literature.J Pediatr Hematol Oncol. 2022 Mar 1;44(2):e612-e615. doi: 10.1097/MPH.0000000000002251. J Pediatr Hematol Oncol. 2022. PMID: 34310473 Review.
Cited by
-
CircRNA circ_0000190 inhibits the progression of multiple myeloma through modulating miR-767-5p/MAPK4 pathway.J Exp Clin Cancer Res. 2019 Feb 6;38(1):54. doi: 10.1186/s13046-019-1071-9. J Exp Clin Cancer Res. 2019. PMID: 30728056 Free PMC article.
-
Patterns of metastases progression- The linear parallel ratio.PLoS One. 2022 Sep 21;17(9):e0274942. doi: 10.1371/journal.pone.0274942. eCollection 2022. PLoS One. 2022. PMID: 36129954 Free PMC article.
-
Clinical Outcomes of TP53 Mutations in Cancers.Cold Spring Harb Perspect Med. 2016 Sep 1;6(9):a026294. doi: 10.1101/cshperspect.a026294. Cold Spring Harb Perspect Med. 2016. PMID: 27449973 Free PMC article. Review.
-
Delineating intra-tumoral heterogeneity and tumor evolution in breast cancer using precision-based approaches.Front Genet. 2023 Aug 17;14:1087432. doi: 10.3389/fgene.2023.1087432. eCollection 2023. Front Genet. 2023. PMID: 37662839 Free PMC article. Review.
-
PROM2 overexpression induces metastatic potential through epithelial-to-mesenchymal transition and ferroptosis resistance in human cancers.Clin Transl Med. 2024 Mar;14(3):e1632. doi: 10.1002/ctm2.1632. Clin Transl Med. 2024. PMID: 38515278 Free PMC article.
References
-
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. - PubMed
-
- Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. - PubMed
-
- Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, Fisher R, McGranahan N, Matthews N, Santos CR, Martinez P, Phillimore B, Begum S, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46:225–233. - PMC - PubMed
-
- Sato Y, Yoshizato T, Shiraishi Y, Maekawa S, Okuno Y, Kamura T, Shimamura T, Sato-Otsubo A, Nagae G, Suzuki H, Nagata Y, Yoshida K, Kon A, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860–867. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

