Should docetaxel be standard of care for patients with metastatic hormone-sensitive prostate cancer? Pro and contra
- PMID: 26002607
- PMCID: PMC4511224
- DOI: 10.1093/annonc/mdv245
Should docetaxel be standard of care for patients with metastatic hormone-sensitive prostate cancer? Pro and contra
Abstract
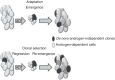
Following the results of the TAX-327 study, questions have been raised as to whether administering chemotherapy to men with prostate cancer before symptomatic disease progression when receiving standard hormonal treatment can improve the duration and quality of patient survival. The GETUG-AFU-15 and CHAARTED studies both assessed the efficacy and tolerability of androgen deprivation therapy (ADT) with or without docetaxel in men with metastatic hormone-naive prostate cancer. Both studies included a mix of patients with de novo metastatic disease (∼75%) and patients who developed metastases following treatment of localized disease. A short course of ADT was allowed in both trials prior to accrual. Key differences between the two studies include the number of patients with high-volume metastases (GETUG-AFU-15: 52%; CHAARTED: 65%) and number of docetaxel cycles (GETUG-AFU-15: up to nine cycles; CHAARTED six cycles). Both studies reported an improvement in progression-free survival with docetaxel plus ADT versus ADT alone. The GETUG-AFU-15 did not find a significant difference in the primary end point of overall survival (OS) {hazard ratio (HR) 0.9 [95% confidence interval (CI) 0.7-1.2]; P = 0.44} for ADT plus docetaxel versus ADT alone. The CHAARTED study met the primary end point of OS [HR 0.61 (95% CI 0.47-0.80); P = 0.0003], and in a subset analysis reported the greatest improvement in OS for patients with high-volume disease [HR 0.60 (95% CI 0.45-0.81); P = 0.0006]. The following article debates the results from the GETUG-AFU-15 and CHAARTED studies and asks whether medical practice should be changed for patients with metastatic hormone-naive prostate cancer based on the results of one positive study.
Keywords: ADT; de novo metastatic; docetaxel; hormone-naive; prostate.
© The Author 2015. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Wu JN, Fish KM, Evans CP et al. . No improvement noted in overall or cause-specific survival for men presenting with metastatic prostate cancer over a 20-year period. Cancer 2013; 120(6): 818–823. - PubMed
-
- Center MM, Jemal A, Lortet-Tieulent J et al. . International variation in prostate cancer incidence and mortality rates. Eur Urol 2012; 61(6): 1079–1092. - PubMed
-
- Patrikidou A, Loriot Y, Eymard J-C et al. . Who dies from prostate cancer? Prostate Cancer Prostatic Dis 2014; 17(4): 348–352. - PubMed
-
- Sweeney C, Carducci MA, Eisenberger MA et al. . Chemohormonal therapy versus hormonal therapy for hormone naive newly metastatic prostate cancer: ECOG-led randomized trial. Ann Oncol 2014; (Suppl 4): Abstr 7560.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

