Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue
- PMID: 26002688
- PMCID: PMC4554812
- DOI: 10.1016/j.heares.2015.04.014
Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue
Abstract
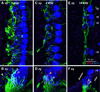
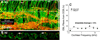
Recent animal work has suggested that cochlear synapses are more vulnerable than hair cells in both noise-induced and age-related hearing loss. This synaptopathy is invisible in conventional histopathological analysis, because cochlear nerve cell bodies in the spiral ganglion survive for years, and synaptic analysis requires special immunostaining or serial-section electron microscopy. Here, we show that the same quadruple-immunostaining protocols that allow synaptic counts, hair cell counts, neuronal counts and differentiation of afferent and efferent fibers in mouse can be applied to human temporal bones, when harvested within 9 h post-mortem and prepared as dissected whole mounts of the sensory epithelium and osseous spiral lamina. Quantitative analysis of five "normal" ears, aged 54-89 yrs, without any history of otologic disease, suggests that cochlear synaptopathy and the degeneration of cochlear nerve peripheral axons, despite a near-normal hair cell population, may be an important component of human presbycusis. Although primary cochlear nerve degeneration is not expected to affect audiometric thresholds, it may be key to problems with hearing in noise that are characteristic of declining hearing abilities in the aging ear.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures








References
-
- Berglund AM, Ryugo DK. Neurofilament antibodies and spiral ganglion neurons of the mammalian cochlea. J Comp Neurol. 1991;306:393–408. - PubMed
-
- Bhatt KA, Liberman MC, Nadol JB., Jr Morphometric analysis of age-related changes in the human basilar membrane. Ann Otol Rhinol Laryngol. 2001;110:1147–1153. - PubMed
-
- Bohne BA, Harding GW. Degeneration in the cochlea after noise damage: primary versus secondary events. Am J Otol. 2000;21:505–509. - PubMed
-
- Brown MC. Morphology of labeled afferent fibers in the guinea pig cochlea. J Comp Neurol. 1987;260:591–604. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

