Distribution and Cellular Uptake of PEGylated Polymeric Particles in the Lung Towards Cell-Specific Targeted Delivery
- PMID: 26002743
- PMCID: PMC4580499
- DOI: 10.1007/s11095-015-1701-7
Distribution and Cellular Uptake of PEGylated Polymeric Particles in the Lung Towards Cell-Specific Targeted Delivery
Abstract
Purpose: We evaluated the role of a poly(ethylene glycol) (PEG) surface coating to increase residence times and alter the cellular fate of nano- and microparticles delivered to the lung.
Methods: Three sizes of PRINT hydrogel particles (80 × 320 nm, 1.5 and 6 μm donuts) with and without a surface PEG coating were instilled in the airways of C57/b6 mice. At time points of 1, 7, and 28 days, BALF and whole lungs were evaluated for the inflammatory cytokine Il-6 and chemokine MIP-2, histopathology, cellular populations of macrophages, dendritic cells (DCs), and granulocytes, and particulate uptake within these cells through flow cytometry, ELISAs, and fluorescent imaging.
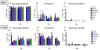
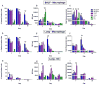
Results: Particles of all sizes and surface chemistries were readily observed in the lung with minimal inflammatory response at all time points. Surface modification with PEGylation was found to significantly increase lung residence times and homogeneous lung distribution, delaying macrophage clearance of all sizes, with the largest increase in residence time observed for 80 × 320 nm particles. Additionally, it was observed that DCs were recruited to the airway following administration of unPEGylated particles and preferentially associated with these particles.
Conclusions: Pulmonary drug delivery vehicles designed with a PEG surface coating can be used to delay particle uptake and promote cell-specific targeting of therapeutics.
Keywords: Microparticle; Nanoparticle; PEGylation; Pulmonary drug delivery.
Figures





References
-
- Pattonand JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nature reviews Drug discovery. 2007;6:67–74. - PubMed
-
- Pulliam B, Sung JC, Edwards DA. Design of nanoparticle-based dry powder pulmonary vaccines. Expert Opin Drug Deliv. 2007;4:651–663. - PubMed
-
- Chow AH, Tong HH, Chattopadhyay P, Shekunov BY. Particle engineering for pulmonary drug delivery. Pharmaceutical research. 2007;24:411–437. - PubMed
-
- Azarmi S, Roa WH, Lobenberg R. Targeted delivery of nanoparticles for the treatment of lung diseases. Advanced drug delivery reviews. 2008;60:863–875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

