Decreased reactivation of a herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) mutant using the in vivo mouse UV-B model of induced reactivation
- PMID: 26002839
- PMCID: PMC4618093
- DOI: 10.1007/s13365-015-0348-9
Decreased reactivation of a herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) mutant using the in vivo mouse UV-B model of induced reactivation
Abstract
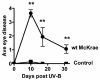
Blinding ocular herpetic disease in humans is due to herpes simplex virus type 1 (HSV-1) reactivations from latency, rather than to primary acute infection. The cellular and molecular immune mechanisms that control the HSV-1 latency-reactivation cycle remain to be fully elucidated. The aim of this study was to determine if reactivation of the HSV-1 latency-associated transcript (LAT) deletion mutant (dLAT2903) was impaired in this model, as it is in the rabbit model of induced and spontaneous reactivation and in the trigeminal ganglia (TG) explant-induced reactivation model in mice. The eyes of mice latently infected with wild-type HSV-1 strain McKrae (LAT((+)) virus) or dLAT2903 (LAT((-)) virus) were irradiated with UV-B, and reactivation was determined. We found that compared to LAT((-)) virus, LAT((+)) virus reactivated at a higher rate as determined by shedding of virus in tears on days 3 to 7 after UV-B treatment. Thus, the UV-B-induced reactivation mouse model of HSV-1 appears to be a useful small animal model for studying the mechanisms involved in how LAT enhances the HSV-1 reactivation phenotype. The utility of the model for investigating the immune evasion mechanisms regulating the HSV-1 latency/reactivation cycle and for testing the protective efficacy of candidate therapeutic vaccines and drugs is discussed.
Keywords: Animal model; Eye; HSV-1; Immunology; LAT; Recurrent herpetic disease; UV-B; Virology.
Figures

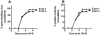
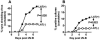
Similar articles
-
The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency.J Virol. 1994 Dec;68(12):8045-55. doi: 10.1128/JVI.68.12.8045-8055.1994. J Virol. 1994. PMID: 7966594 Free PMC article.
-
Prior Corneal Scarification and Injection of Immune Serum are Not Required Before Ocular HSV-1 Infection for UV-B-Induced Virus Reactivation and Recurrent Herpetic Corneal Disease in Latently Infected Mice.Curr Eye Res. 2016 Jun;41(6):747-56. doi: 10.3109/02713683.2015.1061024. Epub 2015 Sep 23. Curr Eye Res. 2016. PMID: 26398722 Free PMC article.
-
Increased neurovirulence and reactivation of the herpes simplex virus type 1 latency-associated transcript (LAT)-negative mutant dLAT2903 with a disrupted LAT miR-H2.J Neurovirol. 2016 Feb;22(1):38-49. doi: 10.1007/s13365-015-0362-y. Epub 2015 Jun 12. J Neurovirol. 2016. PMID: 26069184 Free PMC article.
-
Ocular HSV-1 latency, reactivation and recurrent disease.Semin Ophthalmol. 2008 Jul-Aug;23(4):249-73. doi: 10.1080/08820530802111085. Semin Ophthalmol. 2008. PMID: 18584563 Review.
-
[Battle with herpes for 37 years].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):145-66; discussion 167. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854108 Review. Japanese.
Cited by
-
Docking Proteins Upregulate IL-1β Expression in Lower Esophageal Sphincter Muscle in Esophageal Achalasia.J Clin Med. 2024 May 20;13(10):3004. doi: 10.3390/jcm13103004. J Clin Med. 2024. PMID: 38792545 Free PMC article.
-
Unique molecular signatures of antiviral memory CD8+ T cells associated with asymptomatic recurrent ocular herpes.Sci Rep. 2020 Aug 14;10(1):13843. doi: 10.1038/s41598-020-70673-z. Sci Rep. 2020. PMID: 32796943 Free PMC article.
-
Deletion of Herpes Simplex Virus 1 MicroRNAs miR-H1 and miR-H6 Impairs Reactivation.J Virol. 2020 Jul 16;94(15):e00639-20. doi: 10.1128/JVI.00639-20. Print 2020 Jul 16. J Virol. 2020. PMID: 32295910 Free PMC article.
-
Analysis of the Protective Immunity Induced by Herpes Simplex Virus 1 Strain M3 with an Attenuated Phenotype Due to Mutations in the Viral ul7, ul41, and LAT Genes.Front Microbiol. 2017 Oct 9;8:1958. doi: 10.3389/fmicb.2017.01958. eCollection 2017. Front Microbiol. 2017. PMID: 29062310 Free PMC article.
-
NLRP3, NLRP12, and IFI16 Inflammasomes Induction and Caspase-1 Activation Triggered by Virulent HSV-1 Strains Are Associated With Severe Corneal Inflammatory Herpetic Disease.Front Immunol. 2019 Jul 16;10:1631. doi: 10.3389/fimmu.2019.01631. eCollection 2019. Front Immunol. 2019. PMID: 31367214 Free PMC article.
References
-
- Allen SJ, Hamrah P, Gate D, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, BenMohamed L, Ahmed R, Wechsler SL, Ghiasi H. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus 1. J Virol. 2011;85:4184–97. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI098985/AI/NIAID NIH HHS/United States
- R01 EY013191/EY/NEI NIH HHS/United States
- R01EY024618/EY/NEI NIH HHS/United States
- R56 AI093133/AI/NIAID NIH HHS/United States
- R01 EY019896/EY/NEI NIH HHS/United States
- R01 EY022365/EY/NEI NIH HHS/United States
- R01EY14900/EY/NEI NIH HHS/United States
- R01EY019896/EY/NEI NIH HHS/United States
- R01EY013191/EY/NEI NIH HHS/United States
- R01EY022365/EY/NEI NIH HHS/United States
- 1R56AI098985/AI/NIAID NIH HHS/United States
- R01 EY014900/EY/NEI NIH HHS/United States
- 1R56AI093133/AI/NIAID NIH HHS/United States
- R01 EY024618/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

