The matricellular protein CCN1 controls retinal angiogenesis by targeting VEGF, Src homology 2 domain phosphatase-1 and Notch signaling
- PMID: 26002917
- PMCID: PMC4510592
- DOI: 10.1242/dev.121913
The matricellular protein CCN1 controls retinal angiogenesis by targeting VEGF, Src homology 2 domain phosphatase-1 and Notch signaling
Abstract
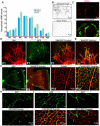
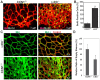
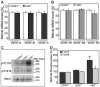
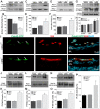
Physiological angiogenesis depends on the highly coordinated actions of multiple angiogenic regulators. CCN1 is a secreted cysteine-rich and integrin-binding matricellular protein required for proper cardiovascular development. However, our understanding of the cellular origins and activities of this molecule is incomplete. Here, we show that CCN1 is predominantly expressed in angiogenic endothelial cells (ECs) at the leading front of actively growing vessels in the mouse retina. Endothelial deletion of CCN1 in mice using a Cre-Lox system is associated with EC hyperplasia, loss of pericyte coverage and formation of dense retinal vascular networks lacking the normal hierarchical arrangement of arterioles, capillaries and venules. CCN1 is a product of an immediate-early gene that is transcriptionally induced in ECs in response to stimulation by vascular endothelial growth factor (VEGF). We found that CCN1 activity is integrated with VEGF receptor 2 (VEGF-R2) activation and downstream signaling pathways required for tubular network formation. CCN1-integrin binding increased the expression of and association between Src homology 2 domain-containing protein tyrosine phosphatase-1 (SHP-1) and VEGF-R2, which leads to rapid dephosphorylation of VEGF-R2 tyrosine, thus preventing EC hyperproliferation. Predictably, CCN1 further brings receptors/signaling molecules into proximity that are otherwise spatially separated. Furthermore, CCN1 induces integrin-dependent Notch activation in cultured ECs, and its targeted gene inactivation in vivo alters Notch-dependent vascular specification and remodeling, suggesting that functional levels of Notch signaling requires CCN1 activity. These data highlight novel functions of CCN1 as a naturally optimized molecule, fine-controlling key processes in physiological angiogenesis and safeguarding against aberrant angiogenic responses.
Keywords: CCN1; Knockout mouse; Matricellular; Retinal angiogenesis; VEGF.
© 2015. Published by The Company of Biologists Ltd.
Figures


References
-
- Bhattacharya R., Kwon J., Wang E., Mukherjee P. and Mukhopadhyay D. (2008). Src homology 2 (SH2) domain containing protein tyrosine phosphatase-1 (SHP-1) dephosphorylates VEGF Receptor-2 and attenuates endothelial DNA synthesis, but not migration. J Mol. Signal. 3, 8 10.1186/1750-2187-3-8 - DOI - PMC - PubMed
-
- Chang K.-H., Chan-Ling T., McFarland E. L., Afzal A., Pan H., Baxter L. C., Shaw L. C., Caballero S., Sengupta N., Calzi S. L. et al. (2007). IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc. Natl. Acad. Sci. USA 104, 10595-10600. 10.1073/pnas.0702072104 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

