CCR9-mediated signaling through β-catenin and identification of a novel CCR9 antagonist
- PMID: 26003048
- PMCID: PMC5512113
- DOI: 10.1016/j.molonc.2015.04.012
CCR9-mediated signaling through β-catenin and identification of a novel CCR9 antagonist
Abstract
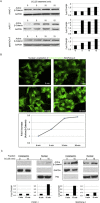
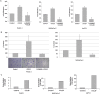
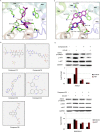
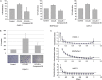
Elevated levels of chemokine receptor CCR9 expression in solid tumors may contribute to poor patient prognosis. In this study, we characterized a novel CCR9-mediated pathway that promotes pancreatic cancer cell invasion and drug resistance, indicating that CCR9 may play a critical role in cancer progression through activation of β-catenin. We noted that the CCL25/CCR9 axis in pancreatic cancer cells induced the activation of β-catenin, which enhanced cell proliferation, invasion, and drug resistance. CCR9-mediated activation of β-catenin and the resulting downstream effects were effectively inhibited by blockade of the PI3K/AKT pathway, but not by antagonism of Wnt. Importantly, we discovered that CCR9/CCL25 increased the lethal dose of gemcitabine, suggesting decreased efficacy of anti-cancer drugs with CCR9 signaling. Through in silico computational modeling, we identified candidate CCR9 antagonists and tested their effects on CCR9/β-catenin regulation of cell signaling and drug sensitivity. When combined with gemcitabine, it resulted in synergistic cytotoxicity. Our results show that CCR9/β-catenin signaling enhances pancreatic cancer invasiveness and chemoresistance, and may be a highly novel therapeutic target.
Keywords: CCL25; CCR9; Drug resistance; Pancreatic cancer; β-catenin.
Copyright © 2015 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- 2012. CCR9 Program – Overview. http://www.chemocentryx.com/about/overview.html.
-
- Al-Aynati, M.M. , Radulovich, N. , Riddell, R.H. , Tsao, M.S. , 2004. Epithelial-cadherin and beta-catenin expression changes in pancreatic intraepithelial neoplasia. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 10, 1235–1240. - PubMed
-
- Amersi, F.F. , Terando, A.M. , Goto, Y. , Scolyer, R.A. , Thompson, J.F. , Tran, A.N. , Faries, M.B. , Morton, D.L. , Hoon, D.S. , 2008. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 14, 638–645. - PMC - PubMed
-
- Ballesteros, J.A. , Weinstein, H. , Stuart, C.S. , 1995. [19] Integrated Methods for the Construction of Three-dimensional Models and Computational Probing of Structure-function Relations in G Protein-coupled Receptors, Methods in Neurosciences Academic Press; 366–428.
-
- Berendsen, H.J.C. , van der Spoel, D. , van Drunen, R. , 1995. GROMACS: a message-passing parallel molecular dynamics implementation. Computer Phys. Commun. 91, 43–56.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

