Inflammatory mechanisms contribute to the neurological manifestations of tuberous sclerosis complex
- PMID: 26003087
- PMCID: PMC4468035
- DOI: 10.1016/j.nbd.2015.04.016
Inflammatory mechanisms contribute to the neurological manifestations of tuberous sclerosis complex
Abstract
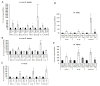
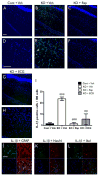
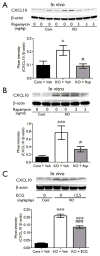
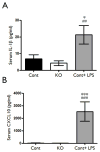
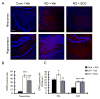
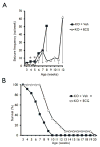
Epilepsy and other neurological deficits are common, disabling manifestations of the genetic disorder, tuberous sclerosis complex (TSC). Brain inflammation has been implicated in contributing to epileptogenesis in acquired epilepsy due to brain injury, but the potential role of inflammatory mechanisms in genetic epilepsies is relatively unexplored. In this study, we investigated activation of inflammatory mediators and tested the effects of anti-inflammatory treatment on epilepsy in the Tsc1-GFAP conditional knock-out mouse model of TSC (Tsc1(GFAP)CKO mice). Real-time quantitative RT-PCR, immunohistochemistry, and Western blotting demonstrated increased expression of specific cytokines and chemokines, particularly IL-1β and CXCL10, in the neocortex and hippocampus of Tsc1(GFAP)CKO mice, which was reversed by treatment with a mammalian target of rapamycin complex 1 (mTORC1) inhibitor. Double-labeling immunohistochemical studies indicated that the increased IL-1β was localized primarily to astrocytes. Importantly, the increase in inflammatory markers was also observed in astrocyte culture in vitro and at 2 weeks of age in Tsc1(GFAP)CKO mice before the onset of epilepsy in vivo, indicating that the inflammatory changes were not secondary to seizures. Epicatechin-3-gallate, an inhibitor of IL-1β and CXCL10, at least partially reversed the elevated cytokine and chemokine levels, reduced seizure frequency, and prolonged survival of Tsc1(GFAP)CKO mice. These findings suggest that mTOR-mediated inflammatory mechanisms may be involved in epileptogenesis in the genetic epilepsy, TSC.
Keywords: Chemokine; Cytokine; Epilepsy; Inflammation; Interleukin; Mice; Seizure; Tuberous sclerosis.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Microglial activation during epileptogenesis in a mouse model of tuberous sclerosis complex.Epilepsia. 2016 Aug;57(8):1317-25. doi: 10.1111/epi.13429. Epub 2016 Jun 6. Epilepsia. 2016. PMID: 27263494 Free PMC article.
-
Cerebral vascular and blood brain-barrier abnormalities in a mouse model of epilepsy and tuberous sclerosis complex.Epilepsia. 2024 Feb;65(2):483-496. doi: 10.1111/epi.17848. Epub 2023 Dec 16. Epilepsia. 2024. PMID: 38049961 Free PMC article.
-
Epileptogenesis and reduced inward rectifier potassium current in tuberous sclerosis complex-1-deficient astrocytes.Epilepsia. 2005 Dec;46(12):1871-80. doi: 10.1111/j.1528-1167.2005.00289.x. Epilepsia. 2005. PMID: 16393152
-
Mechanistic target of rapamycin (mTOR) in tuberous sclerosis complex-associated epilepsy.Pediatr Neurol. 2015 Mar;52(3):281-9. doi: 10.1016/j.pediatrneurol.2014.10.028. Epub 2014 Nov 20. Pediatr Neurol. 2015. PMID: 25591831 Review.
-
A circuitry and biochemical basis for tuberous sclerosis symptoms: from epilepsy to neurocognitive deficits.Int J Dev Neurosci. 2013 Nov;31(7):667-78. doi: 10.1016/j.ijdevneu.2013.02.008. Epub 2013 Feb 26. Int J Dev Neurosci. 2013. PMID: 23485365 Free PMC article. Review.
Cited by
-
Early developmental electroencephalography abnormalities, neonatal seizures, and induced spasms in a mouse model of tuberous sclerosis complex.Epilepsia. 2020 May;61(5):879-891. doi: 10.1111/epi.16495. Epub 2020 Apr 10. Epilepsia. 2020. PMID: 32274803 Free PMC article.
-
mTOR and neuroinflammation in epilepsy: implications for disease progression and treatment.Nat Rev Neurosci. 2024 May;25(5):334-350. doi: 10.1038/s41583-024-00805-1. Epub 2024 Mar 26. Nat Rev Neurosci. 2024. PMID: 38531962 Review.
-
The coding and non-coding transcriptional landscape of subependymal giant cell astrocytomas.Brain. 2020 Jan 1;143(1):131-149. doi: 10.1093/brain/awz370. Brain. 2020. PMID: 31834371 Free PMC article.
-
GABAA receptor function is enhanced by Interleukin-10 in human epileptogenic gangliogliomas and its effect is counteracted by Interleukin-1β.Sci Rep. 2022 Oct 26;12(1):17956. doi: 10.1038/s41598-022-22806-9. Sci Rep. 2022. PMID: 36289354 Free PMC article.
-
Postnatal reduction of tuberous sclerosis complex 1 expression in astrocytes and neurons causes seizures in an age-dependent manner.Epilepsia. 2017 Dec;58(12):2053-2063. doi: 10.1111/epi.13923. Epub 2017 Oct 12. Epilepsia. 2017. PMID: 29023667 Free PMC article.
References
-
- Boer K, Jansen F, Nellist M, Redeker S, van den Ouweland AM, Spliet WG, van Nieuwenhuizen O, Troost D, Crino PB, Aronica E. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res. 2008;78:7–21. - PubMed
-
- Boer K, Crino PB, Gorter JA, Nellist M, Jansen FE, Spliet WG, van Rijen PC, Wittink FR, Breit TM, Troost D, Wadman WJ, Aronica E. Gene expression analysis of tuberous sclerosis complex cortical tubers reveals increased expression of adhesion and inflammatory factors. Brain Pathol. 2010;20:704–719. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

