The Chemical Biology of Molecular Chaperones--Implications for Modulation of Proteostasis
- PMID: 26003923
- PMCID: PMC4569545
- DOI: 10.1016/j.jmb.2015.05.010
The Chemical Biology of Molecular Chaperones--Implications for Modulation of Proteostasis
Abstract
Protein homeostasis (proteostasis) is inextricably tied to cellular health and organismal lifespan. Aging, exposure to physiological and environmental stress, and expression of mutant and metastable proteins can cause an imbalance in the protein-folding landscape, which results in the formation of non-native protein aggregates that challenge the capacity of the proteostasis network (PN), increasing the risk for diseases associated with misfolding, aggregation, and aberrant regulation of cell stress responses. Molecular chaperones have central roles in each of the arms of the PN (protein synthesis, folding, disaggregation, and degradation), leading to the proposal that modulation of chaperone function could have therapeutic benefits for the large and growing family of diseases of protein conformation including neurodegeneration, metabolic diseases, and cancer. In this review, we will discuss the current strategies used to tune the PN through targeting molecular chaperones and assess the potential of the chemical biology of proteostasis.
Keywords: aggregation; heat shock protein; pharmacology; protein folding; small molecule modulators.
Copyright © 2015. Published by Elsevier Ltd.
Figures



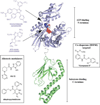
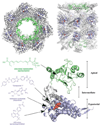
References
-
- Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annual review of biochemistry. 2009;78:959–991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

