Non-invasive mouse models of post-traumatic osteoarthritis
- PMID: 26003950
- PMCID: PMC4577460
- DOI: 10.1016/j.joca.2015.05.009
Non-invasive mouse models of post-traumatic osteoarthritis
Abstract
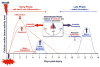
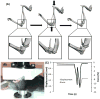
Animal models of osteoarthritis (OA) are essential tools for investigating the development of the disease on a more rapid timeline than human OA. Mice are particularly useful due to the plethora of genetically modified or inbred mouse strains available. The majority of available mouse models of OA use a joint injury or other acute insult to initiate joint degeneration, representing post-traumatic osteoarthritis (PTOA). However, no consensus exists on which injury methods are most translatable to human OA. Currently, surgical injury methods are most commonly used for studies of OA in mice; however, these methods may have confounding effects due to the surgical/invasive injury procedure itself, rather than the targeted joint injury. Non-invasive injury methods avoid this complication by mechanically inducing a joint injury externally, without breaking the skin or disrupting the joint. In this regard, non-invasive injury models may be crucial for investigating early adaptive processes initiated at the time of injury, and may be more representative of human OA in which injury is induced mechanically. A small number of non-invasive mouse models of PTOA have been described within the last few years, including intra-articular fracture of tibial subchondral bone, cyclic tibial compression loading of articular cartilage, and anterior cruciate ligament (ACL) rupture via tibial compression overload. This review describes the methods used to induce joint injury in each of these non-invasive models, and presents the findings of studies utilizing these models. Altogether, these non-invasive mouse models represent a unique and important spectrum of animal models for studying different aspects of PTOA.
Keywords: Articular cartilage; Knee injury; Mouse model; Post-traumatic osteoarthritis (PTOA).
Copyright © 2015 Osteoarthritis Research Society International. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures






Similar articles
-
Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis.Osteoarthritis Cartilage. 2012 Jul;20(7):773-82. doi: 10.1016/j.joca.2012.04.014. Epub 2012 Apr 21. Osteoarthritis Cartilage. 2012. PMID: 22531459
-
Post-traumatic Osteoarthritis in Rabbits Following Traumatic Injury and Surgical Reconstruction of the Knee.Ann Biomed Eng. 2022 Feb;50(2):169-182. doi: 10.1007/s10439-022-02903-6. Epub 2022 Jan 13. Ann Biomed Eng. 2022. PMID: 35028785
-
In vivo fluorescence reflectance imaging of protease activity in a mouse model of post-traumatic osteoarthritis.Osteoarthritis Cartilage. 2014 Oct;22(10):1461-9. doi: 10.1016/j.joca.2014.07.011. Osteoarthritis Cartilage. 2014. PMID: 25278057 Free PMC article.
-
Post-traumatic osteoarthritis after ACL injury.R I Med J (2013). 2014 Nov 3;97(11):25-8. R I Med J (2013). 2014. PMID: 25365816 Review.
-
The role of subchondral bone damage in post-traumatic osteoarthritis.Ann N Y Acad Sci. 2016 Nov;1383(1):58-66. doi: 10.1111/nyas.13261. Epub 2016 Sep 26. Ann N Y Acad Sci. 2016. PMID: 27671712 Review.
Cited by
-
Osteophyte formation after ACL rupture in mice is associated with joint restabilization and loss of range of motion.J Orthop Res. 2017 Mar;35(3):466-473. doi: 10.1002/jor.23252. Epub 2016 Apr 13. J Orthop Res. 2017. PMID: 27031945 Free PMC article.
-
In Vivo Osteocyte Mechanotransduction: Recent Developments and Future Directions.Curr Osteoporos Rep. 2018 Dec;16(6):746-753. doi: 10.1007/s11914-018-0485-1. Curr Osteoporos Rep. 2018. PMID: 30406580 Review.
-
Intra-articular targeting of nanomaterials for the treatment of osteoarthritis.Acta Biomater. 2019 Jul 15;93:239-257. doi: 10.1016/j.actbio.2019.03.010. Epub 2019 Mar 9. Acta Biomater. 2019. PMID: 30862551 Free PMC article. Review.
-
A Reproducible Cartilage Impact Model to Generate Post-Traumatic Osteoarthritis in the Rabbit.J Vis Exp. 2023 Nov 21;(201):10.3791/64450. doi: 10.3791/64450. J Vis Exp. 2023. PMID: 38078617 Free PMC article.
-
Development of Subject Specific Finite Element Models of the Mouse Knee Joint for Preclinical Applications.Front Bioeng Biotechnol. 2020 Oct 15;8:558815. doi: 10.3389/fbioe.2020.558815. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33178671 Free PMC article.
References
-
- Arthritis Foundation. Learn About Osteoarthritis. Retrieved April 15, 2014, from Arthritis Foundation Web site: https://www.arthritis.org/conditions-treatments/disease-center/osteoarth...
-
- Felson DT. Epidemiology of hip and knee osteoarthritis. Epidemiol Rev. 1988;10:1–28. - PubMed
-
- Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–69. - PubMed
-
- Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434–43. - PubMed
-
- Dirschl DR, Marsh JL, Buckwalter JA, Gelberman R, Olson SA, Brown TD, et al. Articular fractures. J Am Acad Orthop Surg. 2004;12(6):416–23. - PubMed
Publication types
MeSH terms
Grants and funding
- 20413/ARC_/Arthritis Research UK/United Kingdom
- 18768/VAC_/Versus Arthritis/United Kingdom
- R01 AG046927/AG/NIA NIH HHS/United States
- R01 AR048182/AR/NIAMS NIH HHS/United States
- R01 AR063757/AR/NIAMS NIH HHS/United States
- P30 AR057235/AR/NIAMS NIH HHS/United States
- AR063757/AR/NIAMS NIH HHS/United States
- AR062603/AR/NIAMS NIH HHS/United States
- AR048182/AR/NIAMS NIH HHS/United States
- 20581/ARC_/Arthritis Research UK/United Kingdom
- AR050245/AR/NIAMS NIH HHS/United States
- AG046927/AG/NIA NIH HHS/United States
- R21 AR063348/AR/NIAMS NIH HHS/United States
- P01 AR050245/AR/NIAMS NIH HHS/United States
- R01 AG015768/AG/NIA NIH HHS/United States
- R21 AR064034/AR/NIAMS NIH HHS/United States
- AR057235/AR/NIAMS NIH HHS/United States
- AG015768/AG/NIA NIH HHS/United States
- BB/I014608/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- AR064034/AR/NIAMS NIH HHS/United States
- 20258/ARC_/Arthritis Research UK/United Kingdom
- K01 AR062603/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

