Concomitant inactivation of the p53- and pRB- functional pathways predicts resistance to DNA damaging drugs in breast cancer in vivo
- PMID: 26004085
- PMCID: PMC5528784
- DOI: 10.1016/j.molonc.2015.04.008
Concomitant inactivation of the p53- and pRB- functional pathways predicts resistance to DNA damaging drugs in breast cancer in vivo
Abstract
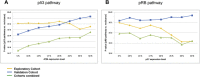
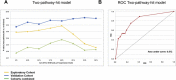
Chemoresistance is the main obstacle to cancer cure. Contrasting studies focusing on single gene mutations, we hypothesize chemoresistance to be due to inactivation of key pathways affecting cellular mechanisms such as apoptosis, senescence, or DNA repair. In support of this hypothesis, we have previously shown inactivation of either TP53 or its key activators CHK2 and ATM to predict resistance to DNA damaging drugs in breast cancer better than TP53 mutations alone. Further, we hypothesized that redundant pathway(s) may compensate for loss of p53-pathway signaling and that these are inactivated as well in resistant tumour cells. Here, we assessed genetic alterations of the retinoblastoma gene (RB1) and its key regulators: Cyclin D and E as well as their inhibitors p16 and p27. In an exploratory cohort of 69 patients selected from two prospective studies treated with either doxorubicin monotherapy or 5-FU and mitomycin for locally advanced breast cancers, we found defects in the pRB-pathway to be associated with therapy resistance (p-values ranging from 0.001 to 0.094, depending on the cut-off value applied to p27 expression levels). Although statistically weaker, we observed confirmatory associations in a validation cohort from another prospective study (n = 107 patients treated with neoadjuvant epirubicin monotherapy; p-values ranging from 7.0 × 10(-4) to 0.001 in the combined data sets). Importantly, inactivation of the p53-and the pRB-pathways in concert predicted resistance to therapy more strongly than each of the two pathways assessed individually (exploratory cohort: p-values ranging from 3.9 × 10(-6) to 7.5 × 10(-3) depending on cut-off values applied to ATM and p27 mRNA expression levels). Again, similar findings were confirmed in the validation cohort, with p-values ranging from 6.0 × 10(-7) to 6.5 × 10(-5) in the combined data sets. Our findings strongly indicate that concomitant inactivation of the p53- and pRB- pathways predict resistance towards anthracyclines and mitomycin in breast cancer in vivo.
Keywords: Breast cancer; Resistance; p53; pRB.
Copyright © 2015 The Authors. Published by Elsevier B.V. All rights reserved.
Figures





References
-
- Alba, E. , Martin, M. , Ramos, M. , Adrover, E. , Balil, A. , Jara, C. , Barnadas, A. , Fernandez-Aramburo, A. , Sanchez-Rovira, P. , Amenedo, M. , Casado, A. , 2004. Multicenter randomized trial comparing sequential with concomitant administration of doxorubicin and docetaxel as first-line treatment of metastatic breast cancer: a Spanish Breast Cancer Research Group (GEICAM-9903) phase III study. J. Clin. Oncol. 22, (13) 2587–2593. - PubMed
-
- Albain, K.S. , Barlow, W.E. , Shak, S. , Hortobagyi, G.N. , Livingston, R.B. , Yeh, I.T. , Ravdin, P. , Bugarini, R. , Baehner, F.L. , Davidson, N.E. , Sledge, G.W. , Winer, E.P. , Hudis, C. , Ingle, J.N. , Perez, E.A. , Pritchard, K.I. , Shepherd, L. , Gralow, J.R. , Yoshizawa, C. , Allred, D.C. , Osborne, C.K. , Hayes, D.F. , Breast Cancer Intergroup of North A, 2010. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 11, (1) 55–65. - PMC - PubMed
-
- Albain, K. , Anderson, S. , Arriagada, R. , Barlow, W. , Bergh, J. , Bliss, J. , Buyse, M. , Cameron, D. , Carrasco, E. , Clarke, M. , Correa, C. , Coates, A. , Collins, R. , Costantino, J. , Cutter, D. , 2012. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100000 women in 123 randomised trials. Lancet. 379, 432–444. - PMC - PubMed
-
- Berge, E.O. , Knappskog, S. , Geisler, S. , Staalesen, V. , Pacal, M. , Borresen-Dale, A.L. , Puntervoll, P. , Lillehaug, J.R. , Lonning, P.E. , 2010. Identification and characterization of retinoblastoma gene mutations disturbing apoptosis in human breast cancers. Mol. Cancer. 9, 173 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

