Ganoderic acid C1 isolated from the anti-asthma formula, ASHMI™ suppresses TNF-α production by mouse macrophages and peripheral blood mononuclear cells from asthma patients
- PMID: 26004313
- PMCID: PMC4635663
- DOI: 10.1016/j.intimp.2015.05.018
Ganoderic acid C1 isolated from the anti-asthma formula, ASHMI™ suppresses TNF-α production by mouse macrophages and peripheral blood mononuclear cells from asthma patients
Abstract
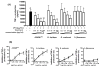
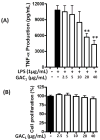
Asthma is a heterogeneous airway inflammatory disease, which is associated with Th2 cytokine-driven inflammation and non-Th2, TNF-α mediated inflammation. Unlike Th2 mediated inflammation, TNF-α mediated asthma inflammation is generally insensitive to inhaled corticosteroids (ICS). ASHMITM, aqueous extract of three medicinal herbs-Ganoderma lucidum (G. lucidum), Sophora flavescens Ait (S. flavescens) and Glycyrrhiza uralensis Fischer (G. uralensis), showed a high safety profile and was clinically beneficial in asthma patients. It also suppresses both Th2 and TNF-α associated inflammation in murine asthma models. We previously determined that G. uralensis flavonoids are the key active compounds responsible for ASHMITM suppression of Th2 mediated inflammation. Until now, there are limited studies on anti-TNF-α compounds presented in ASHMITM. The objective of this study was to isolate and identify TNF-α inhibitory compounds in ASHMITM. Here we report that G. lucidum, but not the other two herbal extracts, S. flavescens or G. uralensis inhibited TNF-α production by murine macrophages; and that the methylene chloride (MC)-triterpenoid-enriched fraction, but not the polysaccharide-enriched fraction, contained the inhibitory compounds. Of the 15 triterpenoids isolated from the MC fraction, only ganoderic acid C1 (GAC1) significantly reduced TNF-α production by murine macrophages (RAW 264.7 cells) and peripheral blood mononuclear cells (PBMCs) from asthma patients. Inhibition was associated with down-regulation of NF-κB expression, and partial suppression of MAPK and AP-1 signaling pathways. Ganoderic acid C1 may have potential for treating TNF-α mediated inflammation in asthma and other inflammatory diseases.
Keywords: ASHMITM; Asthma; Ganoderic acid C1; Ganoderma lucidum; Inflammation; Macrophages; PBMCs; Peripheral blood mononuclear cells; RAW 264.7 cells; TNF-α; Traditional Chinese medicines.
Copyright © 2015. Published by Elsevier B.V.
Figures

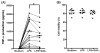

Similar articles
-
Anti-inflammatory Effects of Ganoderma lucidum Triterpenoid in Human Crohn's Disease Associated with Downregulation of NF-κB Signaling.Inflamm Bowel Dis. 2015 Aug;21(8):1918-25. doi: 10.1097/MIB.0000000000000439. Inflamm Bowel Dis. 2015. PMID: 25993687 Free PMC article.
-
Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum.Int Immunopharmacol. 2009 Oct;9(11):1272-80. doi: 10.1016/j.intimp.2009.07.011. Epub 2009 Aug 3. Int Immunopharmacol. 2009. PMID: 19651243
-
Effect of Antiasthma Simplified Herbal Medicine Intervention on neutrophil predominant airway inflammation in a ragweed sensitized murine asthma model.Ann Allergy Asthma Immunol. 2014 Apr;112(4):339-47.e1-2. doi: 10.1016/j.anai.2014.01.021. Ann Allergy Asthma Immunol. 2014. PMID: 24679734 Free PMC article.
-
Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y.Eur J Med Chem. 2019 Jul 15;174:130-141. doi: 10.1016/j.ejmech.2019.04.039. Epub 2019 Apr 20. Eur J Med Chem. 2019. PMID: 31035236 Review.
-
Ganoderma lucidum: Multifaceted mechanisms to combat diabetes through polysaccharides and triterpenoids: A comprehensive review.Int J Biol Macromol. 2024 May;268(Pt 1):131644. doi: 10.1016/j.ijbiomac.2024.131644. Epub 2024 Apr 18. Int J Biol Macromol. 2024. PMID: 38642691 Review.
Cited by
-
An Updated Comprehensive Review of Plants and Herbal Compounds with Antiasthmatic Effect.Evid Based Complement Alternat Med. 2024 Feb 8;2024:5373117. doi: 10.1155/2024/5373117. eCollection 2024. Evid Based Complement Alternat Med. 2024. PMID: 39263346 Free PMC article. Review.
-
Changes in content of triterpenoids and polysaccharides in Ganoderma lingzhi at different growth stages.J Nat Med. 2018 Jun;72(3):734-744. doi: 10.1007/s11418-018-1213-y. Epub 2018 Apr 20. J Nat Med. 2018. PMID: 29679266
-
Sophora flavescens Alkaloids and Corticosteroid Synergistically Augment IL-10/IL-5 Ratio with Foxp3-Gene-Epigenetic Modification in Asthma PBMCs.J Asthma Allergy. 2021 Dec 23;14:1559-1571. doi: 10.2147/JAA.S321616. eCollection 2021. J Asthma Allergy. 2021. PMID: 34992384 Free PMC article.
-
The Effect of Chinese Herbal Medicine Formula mKG on Allergic Asthma by Regulating Lung and Plasma Metabolic Alternations.Int J Mol Sci. 2017 Mar 10;18(3):602. doi: 10.3390/ijms18030602. Int J Mol Sci. 2017. PMID: 28287417 Free PMC article.
-
Cytochrome P450 3A4 suppression by epimedium and active compound kaempferol leads to synergistic anti-inflammatory effect with corticosteroid.Front Pharmacol. 2023 Jan 30;13:1042756. doi: 10.3389/fphar.2022.1042756. eCollection 2022. Front Pharmacol. 2023. PMID: 36793921 Free PMC article.
References
-
- Berry M, Brightling C, Pavord I, Wardlaw A. TNF-alpha in asthma. Curr Opin Pharmacol. 2007;7:279–282. - PubMed
-
- Boh B, Berovic M, Zhang J, Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol Annu Rev. 2007;13:265–301. - PubMed
-
- Brown LL, Rusinova E, Li X. Development of an Efficient Fractionation Procedure for the Identification of Active Compounds in the Complex ASHMI (Anti-Asthma Herbal Medicine Intervention) Formula. Journal of Allergy and Clinical Immunology. 2009;123(2):S256.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

