Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: Role of mitochondrial bioenergetics and energy-sensing mechanism
- PMID: 26004344
- PMCID: PMC4476640
- DOI: 10.1016/j.canlet.2015.05.016
Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: Role of mitochondrial bioenergetics and energy-sensing mechanism
Abstract
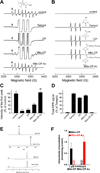
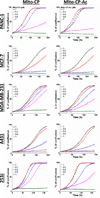
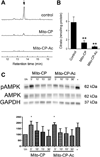
One of the proposed mechanisms for tumor proliferation involves redox signaling mediated by reactive oxygen species such as superoxide and hydrogen peroxide generated at moderate levels. Thus, the antiproliferative and anti-tumor effects of certain antioxidants were attributed to their ability to mitigate intracellular reactive oxygen species (ROS). Recent reports support a role for mitochondrial ROS in stimulating tumor cell proliferation. In this study, we compared the antiproliferative effects and the effects on mitochondrial bioenergetic functions of a mitochondria-targeted cationic carboxyproxyl nitroxide (Mito-CP), exhibiting superoxide dismutase (SOD)-like activity and a synthetic cationic acetamide analog (Mito-CP-Ac) lacking the nitroxide moiety responsible for the SOD activity. Results indicate that both Mito-CP and Mito-CP-Ac potently inhibited tumor cell proliferation. Both compounds altered mitochondrial and glycolytic functions, and intracellular citrate levels. Both Mito-CP and Mito-CP-Ac synergized with 2-deoxy-glucose (2-DG) to deplete intracellular ATP, inhibit cell proliferation and induce apoptosis in pancreatic cancer cells. We conclude that mitochondria-targeted cationic agents inhibit tumor proliferation via modification of mitochondrial bioenergetics pathways rather than by dismutating and detoxifying mitochondrial superoxide.
Keywords: 2-DG; Antioxidant; Mitochondria; ROS; Superoxide; Tumor cell proliferation.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
None
Figures


Similar articles
-
Mitochondria-targeted antioxidant and glycolysis inhibition: synergistic therapy in hepatocellular carcinoma.Anticancer Drugs. 2013 Oct;24(9):881-8. doi: 10.1097/CAD.0b013e32836442c6. Anticancer Drugs. 2013. PMID: 23872912 Free PMC article.
-
Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide.Free Radic Biol Med. 2005 Sep 1;39(5):567-83. doi: 10.1016/j.freeradbiomed.2005.04.016. Free Radic Biol Med. 2005. PMID: 16085176
-
A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: Therapeutic targeting of tumor mitochondria with lipophilic cationic compounds.Redox Biol. 2018 Apr;14:316-327. doi: 10.1016/j.redox.2017.09.020. Epub 2017 Sep 29. Redox Biol. 2018. PMID: 29017115 Free PMC article. Review.
-
Effect of mitochondrially targeted carboxy proxyl nitroxide on Akt-mediated survival in Daudi cells: Significance of a dual mode of action.PLoS One. 2017 Apr 20;12(4):e0174546. doi: 10.1371/journal.pone.0174546. eCollection 2017. PLoS One. 2017. PMID: 28426671 Free PMC article.
-
Reactive Oxygen Species and the Aging Eye: Specific Role of Metabolically Active Mitochondria in Maintaining Lens Function and in the Initiation of the Oxidation-Induced Maturity Onset Cataract--A Novel Platform of Mitochondria-Targeted Antioxidants With Broad Therapeutic Potential for Redox Regulation and Detoxification of Oxidants in Eye Diseases.Am J Ther. 2016 Jan-Feb;23(1):e98-117. doi: 10.1097/MJT.0b013e3181ea31ff. Am J Ther. 2016. PMID: 21048433 Review.
Cited by
-
Mitochondria-targeted drugs stimulate mitophagy and abrogate colon cancer cell proliferation.J Biol Chem. 2018 Sep 21;293(38):14891-14904. doi: 10.1074/jbc.RA117.001469. Epub 2018 Aug 7. J Biol Chem. 2018. PMID: 30087121 Free PMC article.
-
Complex Mitochondrial Dysfunction Induced by TPP+-Gentisic Acid and Mitochondrial Translation Inhibition by Doxycycline Evokes Synergistic Lethality in Breast Cancer Cells.Cells. 2020 Feb 11;9(2):407. doi: 10.3390/cells9020407. Cells. 2020. PMID: 32053908 Free PMC article.
-
Metabolic plasticity in pancreatic cancer: The mitochondrial connection.Mol Metab. 2025 Feb;92:102089. doi: 10.1016/j.molmet.2024.102089. Epub 2024 Dec 28. Mol Metab. 2025. PMID: 39736443 Free PMC article. Review.
-
Magnolia extract is effective for the chemoprevention of oral cancer through its ability to inhibit mitochondrial respiration at complex I.Cell Commun Signal. 2020 Apr 7;18(1):58. doi: 10.1186/s12964-020-0524-2. Cell Commun Signal. 2020. PMID: 32264893 Free PMC article.
-
Triphenyl Phosphine-Functionalized Chitosan Nanoparticles Enhanced Antitumor Efficiency Through Targeted Delivery of Doxorubicin to Mitochondria.Nanoscale Res Lett. 2017 Dec;12(1):158. doi: 10.1186/s11671-017-1931-1. Epub 2017 Feb 28. Nanoscale Res Lett. 2017. PMID: 28249375 Free PMC article.
References
-
- Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. - PubMed
-
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. - PubMed
-
- Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans. 2003;31:1441–1444. - PubMed
-
- Shin SY, Lee JM, Lee MS, Jung H, Lee YH. Targeting cancer cells via the reactive oxygen species-mediated unfolded protein response with a novel synthetic polyphenol conjugate. Clin Cancer Res. 2014;20:4302–4313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

