Progress and problems with the use of suicide genes for targeted cancer therapy
- PMID: 26004498
- PMCID: PMC4758904
- DOI: 10.1016/j.addr.2015.05.009
Progress and problems with the use of suicide genes for targeted cancer therapy
Abstract
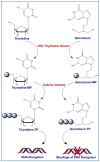
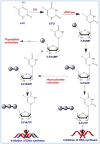
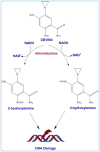
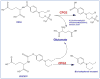
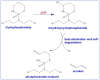
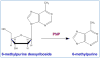
Among various gene therapy methods for cancer, suicide gene therapy attracts a special attention because it allows selective conversion of non-toxic compounds into cytotoxic drugs inside cancer cells. As a result, therapeutic index can be increased significantly by introducing high concentrations of cytotoxic molecules to the tumor environment while minimizing impact on normal tissues. Despite significant success at the preclinical level, no cancer suicide gene therapy protocol has delivered the desirable clinical significance yet. This review gives a critical look at the six main enzyme/prodrug systems that are used in suicide gene therapy of cancer and familiarizes readers with the state-of-the-art research and practices in this field. For each enzyme/prodrug system, the mechanisms of action, protein engineering strategies to enhance enzyme stability/affinity and chemical modification techniques to increase prodrug kinetics and potency are discussed. In each category, major clinical trials that have been performed in the past decade with each enzyme/prodrug system are discussed to highlight the progress to date. Finally, shortcomings are underlined and areas that need improvement in order to produce clinical significance are delineated.
Keywords: Bystander effect; Cancer Gene therapy; Cytosine deaminase; Enzyme prodrug; GDEPT; Ganciclovir; Nitroreductase; Thymidine kinase.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

References
-
- Baban DF, Seymour LW. Control of tumour vascular permeability. Adv Drug Deliv Rev. 1998;34:109–119. - PubMed
-
- DeVita VT, Chu E. A History of Cancer Chemotherapy. Cancer Res. 2008;68:8643–8653. - PubMed
-
- Vandyke K, Fitter S, Dewar AL, Hughes TP, Zannettino AC. Dysregulation of bone remodeling by imatinib mesylate. Blood. 2010;115:766–774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

