hESC Differentiation toward an Autonomic Neuronal Cell Fate Depends on Distinct Cues from the Co-Patterning Vasculature
- PMID: 26004631
- PMCID: PMC4471822
- DOI: 10.1016/j.stemcr.2015.04.013
hESC Differentiation toward an Autonomic Neuronal Cell Fate Depends on Distinct Cues from the Co-Patterning Vasculature
Abstract
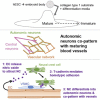
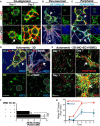
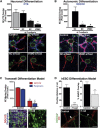
To gain insight into the cellular and molecular cues that promote neurovascular co-patterning at the earliest stages of human embryogenesis, we developed a human embryonic stem cell model to mimic the developing epiblast. Contact of ectoderm-derived neural cells with mesoderm-derived vasculature is initiated via the neural crest (NC), not the neural tube (NT). Neurovascular co-patterning then ensues with specification of NC toward an autonomic fate requiring vascular endothelial cell (EC)-secreted nitric oxide (NO) and direct contact with vascular smooth muscle cells (VSMCs) via T-cadherin-mediated homotypic interactions. Once a neurovascular template has been established, NT-derived central neurons then align themselves with the vasculature. Our findings reveal that, in early human development, the autonomic nervous system forms in response to distinct molecular cues from VSMCs and ECs, providing a model for how other developing lineages might coordinate their co-patterning.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
PSA-NCAM-negative neural crest cells emerging during neural induction of pluripotent stem cells cause mesodermal tumors and unwanted grafts.Stem Cell Reports. 2015 May 12;4(5):821-34. doi: 10.1016/j.stemcr.2015.04.002. Epub 2015 Apr 30. Stem Cell Reports. 2015. PMID: 25937368 Free PMC article.
-
WNT/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate.Development. 2016 Feb 1;143(3):398-410. doi: 10.1242/dev.130849. Development. 2016. PMID: 26839343 Free PMC article.
-
Neural crest and mesoderm lineage-dependent gene expression in orofacial development.Differentiation. 2007 Jun;75(5):463-77. doi: 10.1111/j.1432-0436.2006.00145.x. Epub 2007 Feb 5. Differentiation. 2007. PMID: 17286603
-
Vascular smooth muscle cell differentiation from human stem/progenitor cells.Methods. 2016 May 15;101:85-92. doi: 10.1016/j.ymeth.2015.12.004. Epub 2015 Dec 8. Methods. 2016. PMID: 26678794 Review.
-
Lineage specification in neural crest cell pathfinding.Dev Dyn. 2007 Jan;236(1):1-19. doi: 10.1002/dvdy.20919. Dev Dyn. 2007. PMID: 16894594 Review.
Cited by
-
Vitronectin from brain pericytes promotes adult forebrain neurogenesis by stimulating CNTF.Exp Neurol. 2019 Feb;312:20-32. doi: 10.1016/j.expneurol.2018.11.002. Epub 2018 Nov 6. Exp Neurol. 2019. PMID: 30408465 Free PMC article.
-
Vascularized Organoids: A More Complete Model.Int J Stem Cells. 2021 May 30;14(2):127-137. doi: 10.15283/ijsc20143. Int J Stem Cells. 2021. PMID: 33377457 Free PMC article. Review.
-
The state of the art in stem cell biology and regenerative medicine: the end of the beginning.Pediatr Res. 2018 Jan;83(1-2):191-204. doi: 10.1038/pr.2017.258. Epub 2017 Dec 13. Pediatr Res. 2018. PMID: 29019974 Review.
-
Blood vessel crosstalk during organogenesis-focus on pancreas and endothelial cells.Wiley Interdiscip Rev Dev Biol. 2016 Sep;5(5):598-617. doi: 10.1002/wdev.240. Epub 2016 Jun 21. Wiley Interdiscip Rev Dev Biol. 2016. PMID: 27328421 Free PMC article. Review.
-
Assembly of Human Stem Cell-Derived Cortical Spheroids and Vascular Spheroids to Model 3-D Brain-like Tissues.Sci Rep. 2019 Apr 12;9(1):5977. doi: 10.1038/s41598-019-42439-9. Sci Rep. 2019. PMID: 30979929 Free PMC article.
References
-
- Autiero M., De Smet F., Claes F., Carmeliet P. Role of neural guidance signals in blood vessel navigation. Cardiovasc. Res. 2005;65:629–638. - PubMed
-
- Cheng A., Wang S., Cai J., Rao M.S., Mattson M.P. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev. Biol. 2003;258:319–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

