A comparative analysis of spindle morphometrics across metazoans
- PMID: 26004761
- PMCID: PMC4464779
- DOI: 10.1016/j.cub.2015.04.036
A comparative analysis of spindle morphometrics across metazoans
Abstract
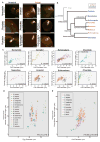
Cell division in all eukaryotes depends on function of the spindle, a microtubule-based structure that segregates chromosomes to generate daughter cells in mitosis or haploid gametes in meiosis. Spindle size adapts to changes in cell size and shape, which vary dramatically across species and within a multicellular organism, but the nature of scaling events and their underlying mechanisms are poorly understood. Cell size variations are most pronounced in early animal development, as egg diameters range from tens of microns up to millimeters across animal phyla, and decrease several orders of magnitude during rapid reductive divisions. During early embryogenesis in the model organisms X. laevis and C. elegans, the spindle scales with cell size [1, 2], a phenomenon regulated by molecules that modulate microtubule dynamics [3-6], as well as by limiting cytoplasmic volume [7, 8]. However, it is not known to what extent spindle scaling is conserved across organisms and among different cell types. Here we show that in a range of metazoan phyla, mitotic spindle length decreased with cell size across an ∼30-fold difference in zygote size. Maximum spindle length varied, but linear spindle scaling occurred similarly in all species once embryonic cell diameter reduced to 140 μm. In contrast, we find that the female meiotic spindle does not scale as closely to egg size, adopting a more uniform size across species that most likely reflects its specialized function. Our analysis reveals that spindle morphometrics change abruptly, within one cell cycle, at the transition from meiosis to mitosis in most animals.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures



References
-
- Hara Y, Kimura A. Cell-size-dependent spindle elongation in the Caenorhabditis elegans early embryo. Curr Biol. 2009;18:1549–1554. - PubMed
-
- Reber SB, Baumgart J, Widlund PO, Pozniakovsky A, Howard J, Hyman AA, Julicher F. XMAP215 activity sets spindle length by controlling the total mass of spindle microtubules. Nat Cell Biol. 2013;9:1116–1122. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

