Efficacy and safety of fixed-combination travoprost 0.004%/timolol 0.5% in patients transitioning from bimatoprost 0.03%/timolol 0.5% combination therapy
- PMID: 26005326
- PMCID: PMC4428365
- DOI: 10.2147/OPTH.S80880
Efficacy and safety of fixed-combination travoprost 0.004%/timolol 0.5% in patients transitioning from bimatoprost 0.03%/timolol 0.5% combination therapy
Abstract
Purpose: To determine the efficacy and safety of fixed-combination travoprost 0.004%/timolol 0.5% preserved with polyquaternium-1 in patients with insufficient response to bimatoprost 0.03%/timolol 0.5% preserved with benzalkonium chloride.
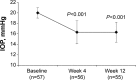
Patients and methods: In this open-label nonrandomized study conducted at 13 European sites, patients with primary open-angle glaucoma or ocular hypertension with insufficient intraocular pressure (IOP) reduction during bimatoprost/timolol therapy were transitioned to travoprost/timolol (DuoTrav(®)) administered every evening for 12 weeks. Change in IOP from baseline to week 12 was assessed in patients who transitioned from fixed-combination bimatoprost/timolol (n=57, primary endpoint). Secondary assessments included change in IOP at week 4, percentage of patients with IOP ≤18 mmHg at weeks 4 and 12, change in Ocular Surface Disease Index and ocular hyperemia scores at week 12, and patient preference. Adverse events were also reported.
Results: IOP change (mean ± SD) from baseline to week 12 was -3.8±1.9 mmHg (P<0.001); results were similar at week 4. Most patients had IOP ≤18 mmHg at weeks 4 and 12 (78.6% and 85.5%, respectively). Mean Ocular Surface Disease Index score was significantly reduced (P<0.001); no significant change in ocular hyperemia score was observed (P=0.197). Treatment-related adverse events included dysgeusia, nausea, paresthesia, myalgia, headache, and eye irritation (n=1 each). Most patients (74.5%) preferred travoprost/timolol over bimatoprost/timolol.
Conclusion: Transition to travoprost/timolol significantly reduced IOP and was well tolerated in patients who had elevated IOP despite bimatoprost/timolol therapy. Polyquaternium-1-preserved travoprost/timolol was preferred over prior treatment with benzalkonium chloride-preserved bimatoprost/timolol.
Keywords: glaucoma; intraocular pressure; preservative; prostaglandin analog; β-blocker.
Figures


Similar articles
-
Fixed Combination of Travoprost and Timolol Maleate Reduces Intraocular Pressure in Japanese Patients with Primary Open-Angle Glaucoma or Ocular Hypertension: A Prospective Multicenter Open-Label Study.Adv Ther. 2015 Sep;32(9):823-37. doi: 10.1007/s12325-015-0246-9. Epub 2015 Sep 30. Adv Ther. 2015. PMID: 26424331 Free PMC article. Clinical Trial.
-
Efficacy and tolerability of benzalkonium chloride-free travoprost in glaucoma patients switched from benzalkonium chloride-preserved latanoprost or bimatoprost.Clin Ophthalmol. 2016 Oct 21;10:2085-2091. doi: 10.2147/OPTH.S112711. eCollection 2016. Clin Ophthalmol. 2016. PMID: 27799736 Free PMC article.
-
Travoprost 0.004%/timolol 0.5% fixed combination in patients transitioning from fixed or unfixed bimatoprost 0.03%/timolol 0.5%.Adv Ther. 2011 Aug;28(8):661-70. doi: 10.1007/s12325-011-0043-z. Epub 2011 Jul 15. Adv Ther. 2011. PMID: 21773673 Clinical Trial.
-
The efficacy of the fixed combination of latanoprost and timolol versus other fixed combinations for primary open-angle glaucoma and ocular hypertension: A systematic review and meta-analysis.PLoS One. 2020 Feb 27;15(2):e0229682. doi: 10.1371/journal.pone.0229682. eCollection 2020. PLoS One. 2020. PMID: 32106236 Free PMC article.
-
Latanoprost : an update of its use in glaucoma and ocular hypertension.Drugs Aging. 2003;20(8):597-630. doi: 10.2165/00002512-200320080-00005. Drugs Aging. 2003. PMID: 12795627 Review.
Cited by
-
Efficacy and tolerability of preservative-free 0.0015% tafluprost in glaucoma patients: a prospective crossover study.BMC Ophthalmol. 2017 Apr 28;17(1):61. doi: 10.1186/s12886-017-0453-z. BMC Ophthalmol. 2017. PMID: 28454526 Free PMC article. Clinical Trial.
-
Maximum Medical Therapy: Brinzolamide/Brimonidine And Travoprost/Timolol Fixed-Dose Combinations In Glaucoma And Ocular Hypertension.Clin Ophthalmol. 2019 Dec 5;13:2411-2419. doi: 10.2147/OPTH.S228777. eCollection 2019. Clin Ophthalmol. 2019. PMID: 31824135 Free PMC article.
-
NOVELTIES IN MEDICAL TREATMENT OF GLAUCOMA.Rom J Ophthalmol. 2015 Apr-Jun;59(2):78-87. Rom J Ophthalmol. 2015. PMID: 26978866 Free PMC article. Review.
-
From Eye Care to Hair Growth: Bimatoprost.Pharmaceuticals (Basel). 2024 Apr 27;17(5):561. doi: 10.3390/ph17050561. Pharmaceuticals (Basel). 2024. PMID: 38794131 Free PMC article. Review.
-
Preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% in patients with open-angle glaucoma and ocular hypertension: results of an open-label observational study.Clin Ophthalmol. 2017 Jun 2;11:1051-1064. doi: 10.2147/OPTH.S128453. eCollection 2017. Clin Ophthalmol. 2017. PMID: 28652689 Free PMC article.
References
-
- Webers CA, Beckers HJ, Nuijts RM, Schouten JS. Pharmacological management of primary open-angle glaucoma: second-line options and beyond. Drugs Aging. 2008;25(9):729–759. - PubMed
-
- Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. - PubMed
LinkOut - more resources
Full Text Sources

